
Organic Chemistry
10th Edition
ISBN: 9781259253379
Publisher: McGraw Hill Higher Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15.4, Problem 3P
Lithium diisopropylamide is often used as a strong base in
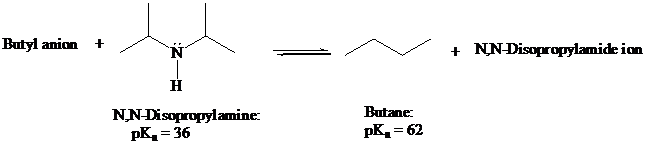
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write a balanced chemical equation for the reaction of Cl2 with benzene to make para-dichlorobenzene in the presence of FeCl3 as a catalyst. Is this an addition or a substitution reaction?
The zinc(II) ion behaves as a Lewis acid. It is found in an enzyme called carboxypeptidase A. This enzyme catalyzed the hydrolysis of C-terminal peptide bonds of proteins. The zinc ion will accept the lone pair of electrons on the oxygen in the peptide linkage. Explain why this Lewis acid behavior would make the carbon atom more susceptible to reaction.
Write the net ionic equation for the equilibrium that is established when calcium cyanide is dissolved in water.
Chapter 15 Solutions
Organic Chemistry
Ch. 15.1 - Each of the following organometallic reagents will...Ch. 15.3 - Write equations showing how you could prepare...Ch. 15.4 - Lithium diisopropylamide is often used as a strong...Ch. 15.5 - Write the structure of the organic product of each...Ch. 15.7 - Prob. 5PCh. 15.8 - Prob. 6PCh. 15.9 - Prob. 7PCh. 15.9 - Like nickel, iron reacts with carbon monoxide to...Ch. 15.9 - Prob. 9PCh. 15.9 - What is the oxidation state of manganese in the...
Ch. 15.9 - Prob. 11PCh. 15.10 - Prob. 12PCh. 15.10 - Prob. 13PCh. 15.11 - Give the structure including stereochemistry of...Ch. 15.11 - Prob. 15PCh. 15.12 - Homogeneous catalytic hydrogenation of the...Ch. 15.12 - Prob. 17PCh. 15.13 - What alkenes are formed from 2-pentene by olefin...Ch. 15.13 - The product of the following reaction was isolated...Ch. 15 - Suggest appropriate methods for preparing each of...Ch. 15 - Prob. 21PCh. 15 - Predict the principal organic product of each of...Ch. 15 - Prob. 23PCh. 15 - Predict the principal organic product of each of...Ch. 15 - Prob. 25PCh. 15 - A different stereoisomer of...Ch. 15 - Prob. 27PCh. 15 - Using phenyllithium and any necessary organic or...Ch. 15 - Prob. 29PCh. 15 - A number of drugs are prepared by reactions in...Ch. 15 - The following conversion was carried out in two...Ch. 15 - Outline syntheses of (a)...Ch. 15 - (S)-(+)-Ibuprofen can be prepared by...Ch. 15 - Like other hydroborations, the reaction of alkynes...Ch. 15 - The sex attractant of the female silkworm has been...Ch. 15 - Prob. 36PCh. 15 - (a) Exaltolide, a musk substance, has been...Ch. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and (Cyclobutadiene)tricarbonyliron...Ch. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Vandium (V) selenide formulaarrow_forwardWrite an equation for the reaction of chloroacetic acid (Ka=1.5103) with trimethylamine (Kb=5.9105) . Calculate the equilibrium constant for the reaction. If 0.10 M solutions of these two species are mixed, what will be their concentrations at equilibrium?arrow_forwardWrite the acidic equilibrium equation for HC₆H₅CO₂arrow_forward
- Silicon and oxygen in extended structures. Silicon and oxygen, building off the basic framework of the simple polyatomic ions, form a huge variety of extended structures. Silicon-oxygen extended chains are found in minerals like asbestos. These cleave (break up) in needle like structures that can be very hazardous. They have a basic repeat unit that has a formula of SiO 3 x (with a negative charge). Draw a structure of a linear chain with this repeat unit. What do you expect will be the charge on a linear SiO 3 structure?arrow_forwardGiven the following data, how do you calculate the pH of 0.62 M solutions of NH4NO3, CH3NH3NO3, and C6H6NH3NO3?arrow_forwardWhat are the 4 basic steps in determining a protein structure using x-ray crystallography?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY