
Concept explainers
Draw structures corresponding to the following names:
(a) 3-Methyl-1, 2-benzenediamine
(b) 1, 3, 5-Benzenetriol
(c) 3-Methyl-2-phenylhexane
(d) o-Aminobenzoic acid
(e) m-Bromophenol
(f) 2, 4, 6-Trinitrophenol (picric acid]
a) 3-methyl-1,2-benzenediamine
Interpretation:
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment ac carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine.
Answer to Problem 19AP
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine is
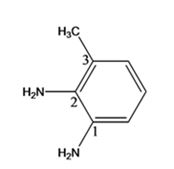
Explanation of Solution
The IUPAC name of the compound, 3-methyl-1,2-benzenediamine, indicates that the compound has a benzene ring with a methyl group on C3 and two amino groups on C1 & C2.
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine

b) 1,3,5-benzenetriol
Interpretation:
The structure of the compound with IUPAC name 1,3,5-benzenetriol is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment as carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 1,3,5-benzenetriol.
Answer to Problem 19AP
The structure of the compound with IUPAC name 1,3,5-benzenetriol is
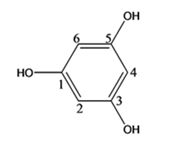
Explanation of Solution
The IUPAC name of the compound, 1,3,5-benzenetriol, indicates that the compound has a benzene ring with three hydroxyl groups on C1, C3 & C5.
The structure of the compound with IUPAC name 1,3,5-benzenetriol is

c) 3-methyl-2-phenylhexane
Interpretation:
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is to be given.
Concept introduction:
Alkyl-substituted benzenes, called arenes, are named in different ways depending on the size of the alkyl group. If the alkyl group is smaller than the ring (six or fewer carbons), the arene is named as an alkyl-substituted benzene. If the alkyl substituent is larger than the ring (seven or more carbons), the arene is named as a phenyl-substitited alkane.
To draw:
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane.
Answer to Problem 19AP
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is
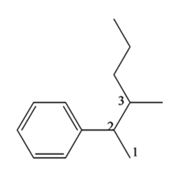
Explanation of Solution
The IUPAC name of the compound, 3-methyl-2-phenyl hexane, indicates that the compound has a six carbon straight chain with a phenyl group on C2 and a methyl group on C3.
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is

d) o-aminobenzoic acid
Interpretation:
The structure of the compound with IUPAC name o-aminobenzoic acid is to be given.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta(m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2- relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name p-aminobenzoic acid.
Answer to Problem 19AP
The structure of the compound with IUPAC name p-aminobenzoic acid is
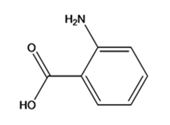
Explanation of Solution
The IUPAC name of the compound, p-aminobenzoic acid, indicates that the compound has a benzene ring with a carboxyl and amino groups in 1,2-relationship.
The structure of the compound with IUPAC name p-aminobenzoicacid is

e) m-bromophenol
Interpretation:
The structure of the compound with IUPAC name m-bromophenol is to be given.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2- relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name m-bromophenol.
Answer to Problem 19AP
The structure of the compound with IUPAC name m-bromophenol is
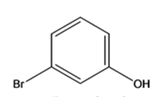
Explanation of Solution
The IUPAC name of the compound, m-bromophenol, indicates that the compound has a benzene ring with a hydroxyl group and bromine atom in 1,3-relationship.
The structure of the compound with IUPAC name m-bromophenol is

f) 2,4,6-trinitrophenol
Interpretation:
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment ac carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 2,4,6-trinitrophenol.
Answer to Problem 19AP
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is
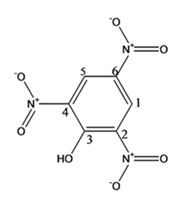
Explanation of Solution
The IUPAC name of the compound, 2,4,6-trinitrophenol, indicates that the compound has a benzenering with a hydroxyl group on C1 and three nitro groups on C2, C4 & C6.
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is

Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry - Owlv2 Access (4 Term)
- Following are the steps in the industrial synthesis of glycerin. Provide structures for all intermediate compounds (AD) and describe the type of mechanism by which each is formed.arrow_forwardCompound A, C5H10O, is one of the basic building blocks of nature. All steroids ans many other naturally occurring compounds are built from compound A. Spectroscopic analysis of A yields the following information. a) how many double bonds and/or rings does A have? b) From the IR spectrum, what is the identity of the oxygen-conataining functional group? c) what kinds of protons are responsible for the NMR absorptions listed d) propose a structure for Aarrow_forwardDefine the Mechanism - Conversion of Carboxylic Acids to Amides with DCCarrow_forward
- Draw the possible stereoisomers of 2-methylocta-4,6-dien-1-amine. Note that E-, Z- isomers of each stereoisomer are also possible and would not be accounted for by the formula above; draw any E- or Z- isomers.arrow_forwardOutline the synthesis of racemic 3-methyl-3-heptanol, Et(Me)C(*)OHn-Bu, starting from alcohols of four carbons or fewer and any inorganic reagents or solvent needed.arrow_forward4.4 Show how the compound thiodiazine - C21H26N2S2 - decomposes anaerobically and how these end products in turn decompose aerobically to stabilized sulfurand nitrogen compounds.arrow_forward
- 3. Obtain acetophenone and acetaldehyde by reaction of glycols with periodic acid. Justify your answer with the reaction mechanism.arrow_forwardplease provide the structure of (1S,2R,4S)-2-amino-4-tert-butylcyclohexanolarrow_forwardIn addition to HCl, what is the product of the reaction of aprimary amine with an acid chloride? Draw the structureof that product and describe its featuresarrow_forward
- Following is an outline of a synthesis of the bronchodilator carbuterol, a beta-2 adrenergic blocker with high selectivity for airway smooth muscle receptors. Q.Suggest a structural relationship between carbuterol and ephedrinearrow_forward(a) Compound Z is a tertiary aromatic amine with the formula, C8H11N. Provide a chemical structure for compound Z. (b)nDraw the structure of the product formed exclusively when nitrous acid reacts with Z.arrow_forwardFollowing is a series of anorexics (appetite suppressants). As you study their structures, you will surely be struck by the sets of characteristic structural features Q. Knowing what you do about the synthesis of amines, including the Ritter reaction , suggest a synthesis for compoundarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

