
Concept explainers
Neuroprotectin D1 (NPD1) is synthesized in the body from highly unsaturated essential fatty acids. NPD1 is a potent natural anti-inflammatory agent.
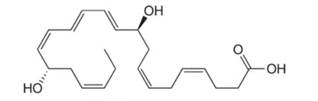
NPD1
a. Label each double bond as conjugated or isolated.
b. Label each double bond as E or Z.
c. For each conjugated system, label the given conformation as s-cis or s-trans.
(a)
Interpretation: Neuroprotectin D1 is synthesized in the body from highly unsaturated essential fatty acids. NPD1 is a potent natural anti-inflammatory agent. The each double bond in NPD1 is to be labeled as conjugated or isolated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
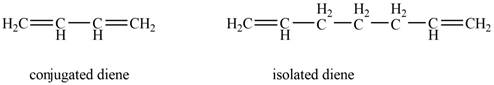
Figure 1
In conjugated diene, electrons in the pi bonds are delocalized, whereas in isolated diene, electrons in the pi bonds are localized.
Answer to Problem 16.11P
The each double bond in NPD1 as conjugated and isolated diene is shown below.
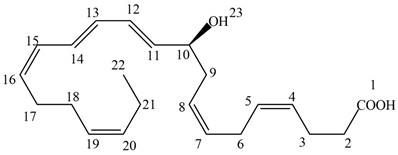
Figure 3
The double bond present between
Explanation of Solution
The given structure of NPD1 is shown below.
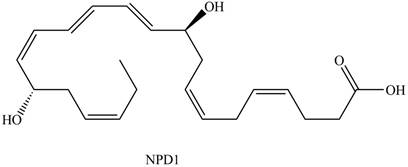
Figure 2
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms. Therefore, carbon atoms of a conjugated double bond are
The each double bond in NPD1 as conjugated and isolated diene is shown below.
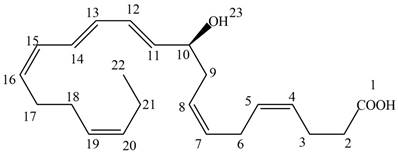
Figure 3
The double bond present between
The each double bond in NPD1 as conjugated or isolated is rightfully stated.
(b)
Interpretation: Neuroprotectin D1 is synthesized in the body from highly unsaturated essential fatty acids. NPD1 is a potent natural anti-inflammatory agent. The each double bond in NPD1 is to be labeled as
Concept introduction: The prefix
Answer to Problem 16.11P
The each double bond in NPD1 as conjugated and isolated diene is shown below.
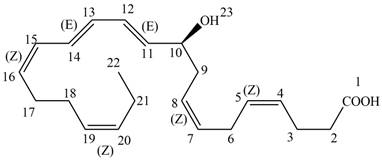
Figure 4
The double bond present between
Explanation of Solution
The given structure of NPD1 is shown below.
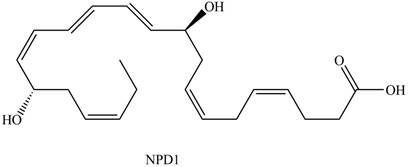
Figure 2
The prefix
The each double bond in NPD1 as conjugated and isolated diene is shown below.
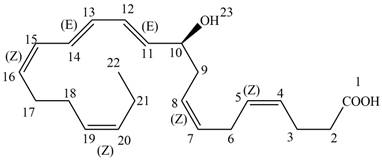
Figure 4
The double bond present between
The each double bond in NPD1 as
(c)
Interpretation: Neuroprotectin D1 is synthesized in the body from highly unsaturated essential fatty acids. NPD1 is a potent natural anti-inflammatory agent. For each conjugated system in NPD1 is to be labeled as
Concept introduction: “If the two double bonds are on the same side of the single bond joining the two double bonds, then it is known as
Answer to Problem 16.11P
The each double bond in NPD1 as conjugated and isolated diene is shown below.
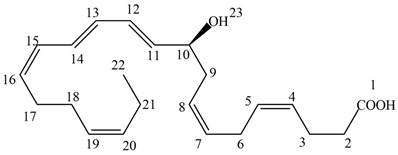
Figure 3
The double bond present between
The double bonds between
Explanation of Solution
The given structure of NPD1 is shown below.
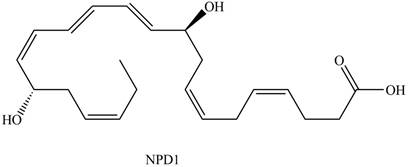
Figure 2
“If the two double bonds are on the same side of the single bond joining the two double bonds, then it is known as
The each double bond in NPD1 as conjugated and isolated diene is shown below.

Figure 3
The double bond present between
The double bonds between
The
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
- (a) which if the structure of trans-1,2-dimethylcyclopentane? (b) which is the most stable conformation of 1-bromo-2-ethylcyclohexane? (c) which is the least stable conformation of 1-bromo-2-ethylcyclohexane? (d) which is the more stable configuration of 1,3-dimethylcyclopentane? *Et = ethylarrow_forwardConsider the tricyclic structure B. (a) Label each substituent on the rings as axial or equatorial. (b) Draw B using chair conformations for each sixmembered ring. (c) Label the atoms on the ring fusions (the carbons that join each set of two rings together) as cis or trans to each other.arrow_forwardClassify each compound as identical to A or its enantiomer.arrow_forward
- Take a look at the butane conformers below. Identify: (a)Which is an anti conformation in Newman? (b)Which is a Gauche conformation? (c)Which is the more stable Sawhorse conformer? (d)Which has the same potential energy/strain with ALS?arrow_forwardLabel each compound as cis or trans. Then draw the second chair conformation.arrow_forwardwhat is the chair conformed and the ring that represents it? which chair conformation is the most stable?arrow_forward
- Draw the two chair conformations of menthol, and identify the more stable conformationarrow_forwardmost stable chair conformation of a Draw the most stable chair conformation of ring of compoundarrow_forwardMenthol is a common additive to dental products and many other everyday items. Here is its structure: a) Draw the two chair conformations of menthol then identify the one that is the most stable. Briefly explain your choice. b) Draw the Newman projection of the most stable chair conformation along the bonds shown in red with the face carbons on the eye side.arrow_forward
- Consider the ball-and-stick model of D, and label E and F as either identical to D or an enantiomer of Darrow_forwardLabel each stereogenic center as R or S (parts d, e and f please)arrow_forwardClassify each conformation as staggered or eclipsed around the indicated bond, and rank the conformations in order of increasing stability.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

