1) Concept:
To calculate the pH of a weak acid with a strong base, we need to use reaction stoichiometry and RICE table. First, we will calculate the equivalence point from the volume and molarity of base and acid. We will calculate the pH at equivalence point by using the RICE table. At the equivalence point, moles of phenol and NaOH are equal, so the only species present is phenoxide ion. Using the hydrolysis of phenoxide ions and RICE table, we can find the pH at the equivalence point. Suitable indicator for this titration is decided by comparing the pH at equivalence point and the pH range of indicators given in Figure 16.5. We will sketch the titration curve based on the pH of the solution and mL of a strong base added to the weak acid.
2) Formulae:
i) pKa= -logKa
ii) pH=pKa+log[base][acid]
iii) n=M×V
iv) Kb=KwKa
3) Given:
i) Volume of phenol = 21.5 mL=0.0215 L
ii) Molarity of phenol =0.120 M
iii) pKa of phenol = 9.89
iv) Molarity of NaOH=0.250M
4) Calculations:
a) Calculating pH of the solution at the equivalence point:
C6H5-OH aq+NaOH aq→ C6H5-ONaaq+H2O
Step 1)
Finding the volume of NaOH required for the equivalence point by finding the moles of phenol:
At the equivalence point, moles of phenol = moles of NaOH
nphenol=Mphenol×Vphenol=0.120 M ×0.0215 L=0.00258 mol phenol
At the equivalence point, moles of phenol and NaOH are equal. So, there are 0.00258 mol of NaOH.
Calculating the volume of NaOH at the equivalence point from the given molarity and calculated moles as
nNaOH=MNaOH×VNaOH
nNaOHMNaOH=VNaOH=0.00258 mol 0.250 M=0.01032 L
So, at the equivalence point, the volume of NaOH required is 0.01032 L=10.32 mL.
So, the total volume of solution is =21.5+10.32=31.85 mL=0.03185 L
At the equivalence point, sodium salt of phenol is formed, and its moles will be equal to moles of phenol and NaOH consumed =0.00258 mol
Calculating the molarity of sodium phenoxide:
M=nV= 0.00258 mol0.03185 L=0.08108 M
Step 2)
Creating an RICE table for the dissociation of sodium phenoxide (C6H5-ONa):
| C6H5-ONa aq+H2Oaq⇌C6H5-OH aq+OH-aq |
| RICE |
C6H5-ONa |
C6H5-OH |
OH- |
| Initial (M) |
0.08108 M |
0.0 |
0.0 |
| Change (M) |
-x |
+x |
+x |
| Equilibrium (M) |
(0.08108-x) |
x |
x |
pKa for phenol =9.89
Ka phenol = 10-pKa=10-9.89= 1.288 ×10-10
Kb=KwKa=1×10-141.288 ×10-10=7.764 ×10-5
Writing the Kb expression for the reaction and calculating pH:
Kb=7.764 ×10-5= x20.08108-x
x2= 7.764 ×10-5×0.08108=6.292 ×10-6
x= 6.292 ×10-6=0.002508 M=OH-
pOH= -logOH-= -log0.00250876=2.600
pH=14-2.600=11.40
The calculated pH at the equivalence point comes out to be 11.40, which falls under the basic range of pH. This is reasonable since the salt of phenol (weak acid) and NaOH(strong base), will be basic.
b) Choosing the indicator, which is suitable for the titration referring to figure 16.5:
A pH indicator is useful within a range of one pH unit above and below the pKa value of the indicator. The pH at the equivalence point is 11.60. The indicator Alizarin yellow R is in the range 10-12, which will be the suitable indicator for this titration.
c) Calculating the pH for the titration after volume of NaOH added to 21.5 mL of weak acid:
The calculated volume of NaOH at the equivalence point is 10.32 mL, so, before reaching this volume, the NaOH would be the limiting reactant, and we can use the Henderson-Hasselbalch equation to find the pH.
1) Addition of 0.0 mL NaOH:
At 0.0mL NaOH added, the pH of the phenol is solely because of phenol. The dissociation reaction of phenol is written as
| C6H5-OH aq +H2Oaq⇌ C6H5-O- aq + H3O+aq |
| RICE |
C6H5-OH(M) |
C6H5-O-(M) |
H3O+(M) |
| Initial (M) |
0.120M |
0.0 |
0.0 |
| Change (M) |
-x |
+x |
+x |
| Equilibrium (M) |
(0.120-x) |
x |
x |
Ka= 1.288 ×10-10= x2(0.120-M)
x2=1.288 ×10-10 ×0.120=1.5456 ×10-11
x= 1.5456 ×10-11=3.9314 ×10-6=H3O+
pH= -logH3O+ = -log3.9314 ×10-6 =5.41
So, the pH, when 0.0 mLNaOH were added, is 5.41
2) Addition of 2.5 mL NaOH:
Moles of phenol:nphenol=Mphenol×Vphenol=0.120 M ×0.0215 L=0.00258 mol phenol
Moles of NaOH for 2.5 mL=0.0025L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.0025 L= 0.000625 mol NaOH
We create a modified RICE table to determine how many moles of acid remain and how many moles of conjugate base have been produced:
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.000625 |
0.0 |
| Change (mol) |
-0.000625 |
-0.000625 |
+0.000625 |
| Final (mol) |
0.001955 |
0 |
0.000625 |
The total sample volume is 21.5 mL + 2.5 mL = 24.0 mL or 0.0240 L, and the concentrations of the base and conjugate acid is
C6H5-OH= 0.001955 mol0.0240 L=0.08146 M
C6H5-O-= 0.000625 mol0.0240 L=0.02604 M
Applying the Henderson-Hasselbalch equation for the above buffer system formed as
pH=pKa+log[base][acid]
pH=9.89+log[0.02604][0.08146]
pH=9.39
3) Addition of 5.0 mL NaOH:
Moles of phenol:nphenol=Mphenol×Vphenol=0.120 M ×0.0215 L=0.00258 mol phenol
Moles of NaOH for 5.0 mL=0.005L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.005 L= 0.00125 mol NaOH
We create a modified RICE table to determine how many moles of acid remain and how many moles of conjugate base have been produced:
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.000125 |
0.0 |
| Change (mol) |
-0.00125 |
-0.00125 |
+0.00125 |
| Final (mol) |
0.00133 |
0 |
0.00125 |
The total sample volume is 21.5 mL + 5.0 mL = 26.5 mL or 0.0265 L, and the concentrations of the base and conjugate acid is
C6H5-OH= 0.001330 mol0.0265 L=0.05019 M
C6H5-O-= 0.00125 mol0.0265 L=0.04717 M
Applying the Henderson-Hasselbalch equation for the above buffer system formed as
pH=pKa+log[base][acid]
pH=9.89+log[0.04717][0.05019]
pH=9.86
4) Addition of 7.5 mL NaOH:
Moles of phenol:nphenol=Mphenol×Vphenol=0.120 M ×0.0215 L=0.00258 mol phenol
Moles of NaOH for 7.5 mL=0.0075L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.0075L = 0.001875 mol NaOH
We create a modified RICE table to determine how many moles of acid remains and how many moles of conjugate base have been produced.
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.001875 |
0.0 |
| Change (mol) |
-0.001875 |
-0.001875 |
+0.001875 |
| Final (mol) |
0.000705 |
0 |
0.001875 |
The total sample volume is 21.5 mL + 7.5 mL = 29.0 mL or 0.029 L, and the concentrations of the base and conjugate acid is
C6H5-OH= 0.000705 mol0.029 L=0.02431 M
C6H5-O-= 0.001875 mol0.029 L=0.06466 M
Applying the Henderson-Hasselbalch equation for the above buffer system formed as
pH=pKa+log[base][acid]
pH=9.89+log[0.06466 ][0.02431]
pH=10.31
5) Addition of 9.8 mL NaOH:
Moles of phenol:nphenol=Mphenol×Vphenol=0.120 M ×0.0215 L=0.00258 mol phenol
Moles of NaOH for 9.8 mL=0.0098L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.0098L = 0.00245 mol NaOH
We create a modified RICE table to determine how many moles of acid remain and how many moles of conjugate base have been produced:
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.00245 |
0.0 |
| Change (mol) |
-0.00245 |
-0.00245 |
+0.00245 |
| Final (mol) |
0.000130 |
0 |
0.00245 |
The total sample volume is 21.5 mL + 9.8 mL = 31.3 mL or 0.0313 L, and the concentrations of the base and conjugate acid is
C6H5-OH= 0.000130 mol0.0313 L=0.00415 M
C6H5-O-= 0.00245 mol0.0313 L=0.07827 M
Applying the Henderson-Hasselbalch equation for the above buffer system formed as
pH=pKa+log[base][acid]
pH=9.89+log[0.07827 ][0.00415]
pH=11.17
6) Addition of 10 mL NaOH:
Moles of phenol:nphenol=Mphenol×Vphenol=0.120 M ×0.0215 L=0.00258 mol phenol
Moles of NaOH for 10 mL=0.01L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.010L = 0.0025 mol NaOH
We create a modified RICE table to determine how many moles of acid remain and how many moles of conjugate base have been produced:
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.0025 |
0.0 |
| Change (mol) |
-0.0025 |
-0.0025 |
+0.0025 |
| Final (mol) |
0.00008 |
0 |
0.0025 |
The total sample volume is 21.5 mL + 10.0 mL = 31.5 mL or 0.0315 L, and the concentrations of the base and conjugate acid is
C6H5-OH= 0.000080 mol0.0315 L=0.00254 M
C6H5-O-= 0.0025 mol0.0315 L=0.07937 M
Applying the Henderson-Hasselbalch equation for the above buffer system formed as
pH=pKa+log[base][acid]
pH=9.89+log[0.07937 ][0.00254]
pH=11.39
7) Addition of 10.2 mL NaOH:
Moles of phenol:nphenol=Mphenol×Vphenol=0.120 M ×0.0215 L=0.00258 mol phenol
Moles of NaOH for 10.2 mL=0.0102 L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.0102L = 0.00255 mol NaOH
We create a modified RICE table to determine how many moles of acid remain and how many moles of conjugate base have been produced:
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.00255 |
0.0 |
| Change (mol) |
-0.00255 |
-0.00255 |
+0.00255 |
| Final (mol) |
0.00003 |
0 |
0.00255 |
The total sample volume is 21.5 mL + 10.2 mL = 31.7 mL or 0.0317 L, and the concentrations of the base and conjugate acid is
C6H5-OH= 0.00003 mol0.0317 L=0.00095 M
C6H5-O-= 0.00255 mol0.0317 L=0.08044 M
Applying the Henderson-Hasselbalch equation for the above buffer system formed as
pH=pKa+log[base][acid]
pH=9.89+log[0.08044 ][0.00095]
pH=11.82
8) Addition of 10.6 mL NaOH:
The contribution of OH- ions from sodium pehnoxide is much smaller as compared to the contribution of OH- ions from NaOH. The pH of the solution will be governed by solely NaOH.
Moles of NaOH for 10.6 mL=0.0106 L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.0106L = 0.00265 mol NaOH
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.00265 |
0.0 |
| Change (mol) |
-0.00258 |
-0.00258 |
+0.00258 |
| Final (mol) |
0 |
0.00007 |
0.00258 |
Total volume of solution = 21.5+10.6=32.1 mL=0.0321L
Molarity of OH- is
OH-=0.00007 mol0.0321 L = 0.0021807 M
pOH= -logOH-= -log0.00218065=2.6614
pH=14-2.6614=11.34
9) Addition of 10.8 mL NaOH
10.8 mLNaOH
Moles of NaOH for 10.8 mL=0.0108 L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.0108L = 0.00270 mol NaOH
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.00270 |
0.0 |
| Change (mol) |
-0.00258 |
-0.00258 |
+0.00258 |
| Final (mol) |
0 |
0.00012 |
0.00258 |
Total volume of solution = 21.5+10.8=32.3 mL=0.0323L
Molarity of OH- is
OH-=0.00012 mol0.0323 L = 0.003715 M
pOH= -logOH-= -log0.003715 =2.4300
pH=14-2.4300=11.57
10) Addition of 11 mL NaOH
Moles of NaOH for 11 mL=0.011 L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.011L = 0.00275 mol NaOH
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.00275 |
0.0 |
| Change (mol) |
-0.00258 |
-0.00258 |
+0.00258 |
| Final (mol) |
0 |
0.00017 |
0.00258 |
Total volume of solution = 21.5+11=32.5 mL=0.0325L
Molarity of OH- is
OH-=0.00017 mol0.0325 L = 0.005231 M
pOH= -logOH-= -log 0.005231=2.2814
pH=14-2.2814=11.72
11) Addition of 12.5 mL NaOH
Moles of NaOH for 12.5 mL=0.0125 L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.0125L = 0.003125 mol NaOH
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.003125 |
0.0 |
| Change (mol) |
-0.00258 |
-0.00258 |
+0.00258 |
| Final (mol) |
0 |
0.000545 |
0.00258 |
Total volume of solution = 21.5+12.5=34 mL=0.034L
Molarity of OH- is
OH-=0.000545 mol0.034 L = 0.016029 M
pOH= -logOH-= -log0.016029 =1.79508
pH=14-1.79508=12.20
12) Addition of 15 mL NaOH
Moles of NaOH for 15 mL=0.0150 L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.0150L = 0.00375 mol NaOH
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.00375 |
0.0 |
| Change (mol) |
-0.00258 |
-0.00258 |
+0.00258 |
| Final (mol) |
0 |
0.00117 |
0.00258 |
Total volume of solution = 21.5+15=36.5 mL=0.0365L
Molarity of OH- is
OH-=0.00117 mol0.0365 L = 0.03205 M
pOH= -logOH-= -log0.03205=1.4941
pH=14-1.4941=12.51
13) Addition of 17.5 mL NaOH:
Moles of NaOH for 17.5 mL=0.0175 L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.0175L = 0.004375 mol NaOH
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.004375 |
0.0 |
| Change (mol) |
-0.00258 |
-0.00258 |
+0.00258 |
| Final (mol) |
0 |
0.001795 |
0.00258 |
Total volume of solution = 21.5+17.5=39 mL=0.039 L
Molarity of OH- is
OH-=0.001795 mol0.039 L = 0.046026 M
pOH= -logOH-= -log0.046026=1.3370
pH=14-1.3370=12.66
14) Addition of 20 mLNaOH
Moles of NaOH for 20 mL=0.020L volume:
nNaOH=MNaOH×VNaOH=0.250 M ×0.020L = 0.005 mol NaOH
| C6H5-OH aq + NaOHaq→ C6H5-O- Na+aq +H2Ol C6H5-OH(Mol) NaOH(mol) C6H5-O-(mol) |
| Initial (mol) |
0.00258 |
0.005 |
0.0 |
| Change (mol) |
-0.00258 |
-0.00258 |
+0.00258 |
| Final (mol) |
0 |
0.00242 |
0.00258 |
Total volume of solution = 21.5+20=41.5 mL=0.0415L
Molarity of OH- is
OH-=0.00242 mol0.0415 L = 0.058313 M
pOH= -logOH-= -log0.058313=1.2342
pH=14-1.2342=12.77
d) Graph of pH vs mL of NaOH added is
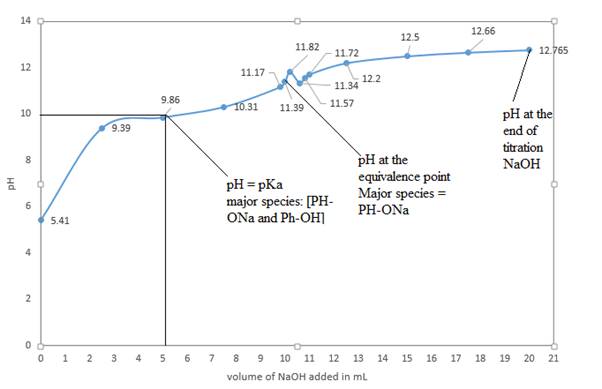
The above curve is a plot of pH vs. mL of NaOH added.
The volume of NaOH at half equivalence point is 10.3 mL, so the half equivalence point is around 5.15 mL of NaOH. If we draw a vertical line from 5.15 mL to the curve, it meets at around 9.89. So, the pH at half equivalence point is 9.89. This value matches with the given pKa of 9.89. At the half equivalence point, the conjugate base formed and the acid present are equal in concentrations, so the Henderson-Hasselbalch equation reduces to pH=pKa.
So, at half equivalence point, the major species would be C6H5-ONa and C6H5-OH.
At the equivalence point, moles of acid initially present are equal to moles of added base. So, there will not be phenol or NaOH present at the equivalence point. The salt of phenol, that is, sodium phenoxide, C6H5-ONa, is a major species that is present at the equivalence point.
At the end of the titration, the only major species present will be NaOH.
Conclusion:
In the titration of a weak acid and strong base, the resultant salt formed is a basic salt. Hence, the pH at the equivalence point of the titration is greater than 7.



 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY




