
Concept explainers
(a)
Interpretation: The diene that has the larger heat of hydrogenation is to be identified.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
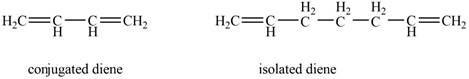
Figure 1
The stability of conjugated diene is more than isolated diene. Due to which the heat of hydrogenation is smaller for conjugated diene, whereas heat of hydrogenation for isolated diene is larger.
(b)
Interpretation: The diene that has the larger heat of hydrogenation is to be identified.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
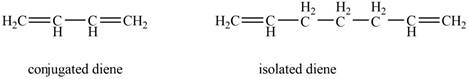
Figure 1
The stability of conjugated diene is more than isolated diene. Due to which the heat of hydrogenation is smaller for conjugated diene, whereas heat of hydrogenation for isolated diene is larger.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
ORGANIC CHEMISTRY SOLUTIONS MANUAL
- (a) Draw a stepwise mechanism for the conversion of A to B. (b) What product would be formed if C was exposed to similar reaction conditions?arrow_forwardWhich is an energy diagram for a concerted reaction (SN2 and E2)? A B C Darrow_forwardAddition of HCl to alkene X forms two alkyl halides Y and Z. (A) Label Y and Z as a kinetic or thermodynamic product and explain why. (B) Explain why the addition of HCl occurs at the exocyclic C=C, rather than the other C=Carrow_forward
- Rank the compounds in each set from most to least reactive in an EAS reactionarrow_forwardWhich is the major product?a. Ab. Neither product would likely formc. Barrow_forwardWrite down the reduction reaction of each molecule using appropriate reagents, listing the follo compounds according to their of reductionarrow_forward
- Which carbocation would be lowest energy? Options: A B C Darrow_forwardDevise a stepwise synthesis of attached compound from dicyclopentadieneusing a Diels–Alder reaction as one step. You may also use organiccompounds having ≤ 4 C's, and any required organic or inorganicreagents.arrow_forwardWhich group in following pair is assigned the higher priority? −H, −Darrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
