
Concept explainers
Draw the products formed when each diene is treated with one equivalent of
a. 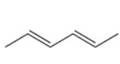 b.
b. 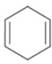 c.
c. 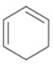 d.
d. 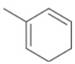
(a)
Interpretation: The product formed by the reaction of given diene with one equivalent of
Concept introduction: Diene is a hydrocarbon that contains two
Answer to Problem 16.16P
The product formed by the reaction of given diene with one equivalent of
Explanation of Solution
Conjugated dienes undergoes electrophilic addition to gives a mixture of products that is
Markovnikov addition of
The given diene is shown below.

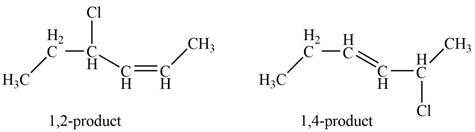
Figure 1
The given diene is a conjugated diene. The attack of
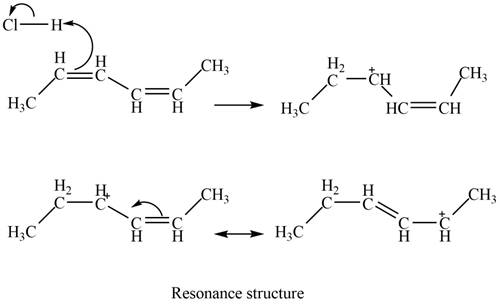
Figure 2
In the next step, chlorine as a nucleophile will attack on the carbocation to give constitutional isomers. Thus, the product formed by the reaction of given diene with one equivalent of
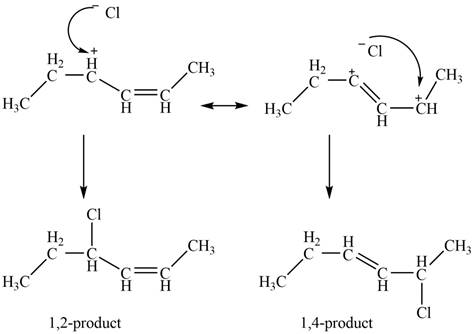
Figure 3
The product formed by the reaction of given diene with one equivalent of
(b)
Interpretation: The product formed by the reaction of given diene with one equivalent of
Concept introduction: Diene is a hydrocarbon that contains two
Answer to Problem 16.16P
The product formed by the reaction of given diene with one equivalent of
Explanation of Solution
Conjugated dienes undergoes electrophilic addition to gives a mixture of products that is
Markovnikov’s rule states that the positive part of acid attached to that carbon atom in
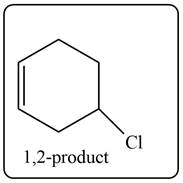
The given diene is shown below.
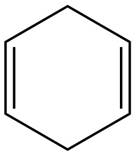
Figure 4
The given diene is an isolated diene. The attack of

Figure 5
In the next step, chlorine as a nucleophile will attack on the carbocation to form desired product. Thus, the product formed by the reaction of given diene with one equivalent of
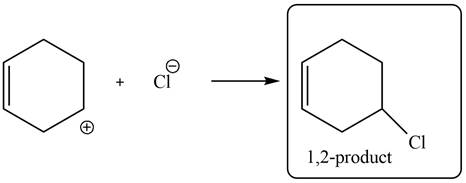
Figure 6
The product formed by the reaction of given diene with one equivalent of
(c)
Interpretation: The product formed by the reaction of given diene with one equivalent of
Concept introduction: Diene is a hydrocarbon that contains two
Answer to Problem 16.16P
The product formed by the reaction of given diene with one equivalent of
Explanation of Solution
Conjugated dienes undergoes electrophilic addition to gives a mixture of products that is
Markovnikov addition of
The given diene is shown below.
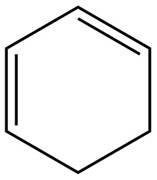
Figure 7
The given diene is a conjugated diene. The attack of
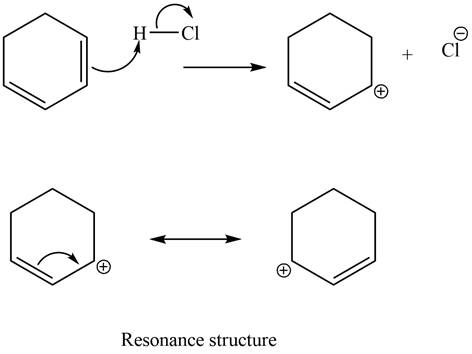
Figure 8
In the next step, chlorine as a nucleophile will attack on the carbocation to give constitutional isomers. Thus, the product formed by the reaction of given diene with one equivalent of
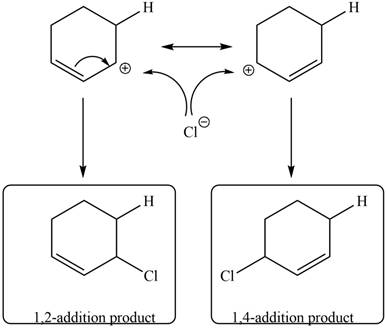
Figure 9
The product formed by the reaction of given diene with one equivalent of
(d)
Interpretation: The product formed by the reaction of given diene with one equivalent of
Concept introduction: Diene is a hydrocarbon that contains two
Answer to Problem 16.16P
The product formed by the reaction of given diene with one equivalent of
Explanation of Solution
Conjugated dienes undergoes electrophilic addition to gives a mixture of products that is
Markovnikov addition of
The given diene is shown below.
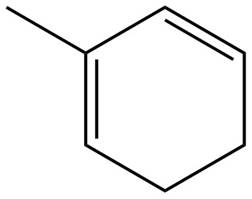
Figure 10
The given diene is a conjugated diene. The attack of
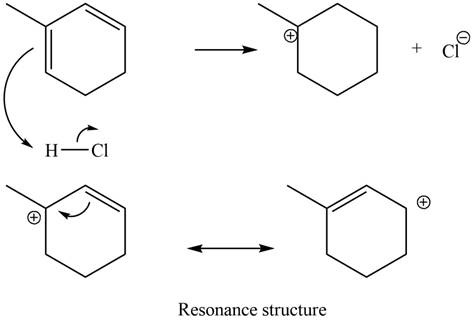
Figure 11
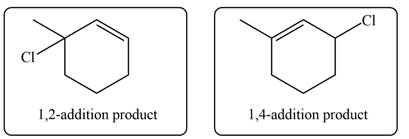
In the next step, chlorine as a nucleophile will attack on the carbocation to give constitutional isomers. Thus, the product formed by the reaction of given diene with one equivalent of
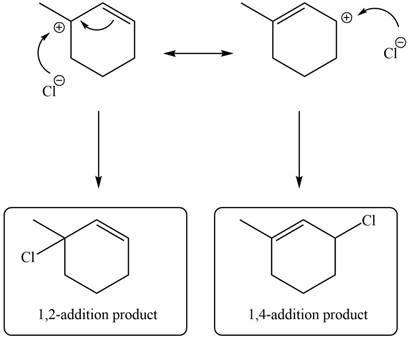
Figure 12
The product formed by the reaction of given diene with one equivalent of
Want to see more full solutions like this?
Chapter 16 Solutions
Connect Access Card Two Year for Organic Chemistry
- Write the appropriate reagent over each arrow.arrow_forwardDimethyl cyclopropanes can be prepared by the reaction of an α, β-unsaturated carbonyl compound X with two equivalents of a Wittigreagent Y. Draw a stepwise mechanism for this reaction.arrow_forwardDraw a stepwise mechanism and all stereoisomers formed following reaction.arrow_forward
- Draw a stepwise mechanism for the dienone–phenol rearrangement, a reaction that forms alkyl-substituted phenols from cyclohexadienones.arrow_forwardDraw the products of each reaction. (a) and (b)arrow_forwardDraw a stepwise mechanism for the attached reaction, which involvesboth a Diels–Alder reaction and a nucleophilic acyl substitution.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
