
(a)
Interpretation: The given diene is to be classified as isolated or conjugated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
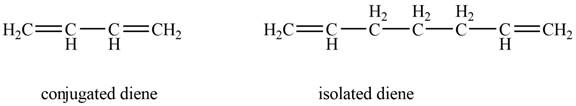
Figure 1
Answer to Problem 16.1P
The given diene is a conjugated diene.
Explanation of Solution
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The given diene is shown below.
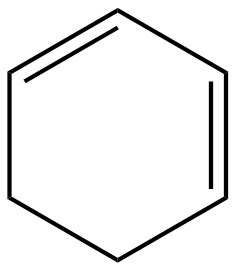
Figure 2
In the given diene, two double bonds are separated by a single bond. Thus, the given diene is a conjugated diene.
The given diene is a conjugated diene.
(b)
Interpretation: The given diene is to be classified as isolated or conjugated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
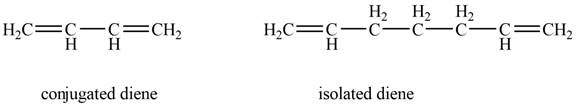
Figure 1
Answer to Problem 16.1P
The given diene is an isolated diene.
Explanation of Solution
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The given diene is shown below.
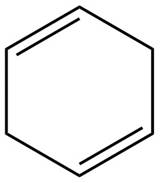
Figure 3
In the given diene, two double bonds are separated by a two sigma bonds. Thus, the given diene is an isolated diene.
The given diene is an isolated diene.
(c)
Interpretation: The given diene is to be classified as isolated or conjugated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
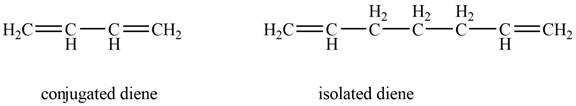
Figure 1
Answer to Problem 16.1P
The given diene is a conjugated diene.
Explanation of Solution
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The given diene is shown below.
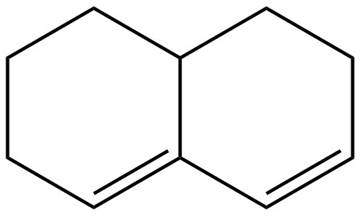
Figure 4
In the given diene, two double bonds are separated by a single bond. Thus, the given diene is a conjugated diene.
The given diene is a conjugated diene.
(d)
Interpretation: The given diene is to be classified as isolated or conjugated.
Concept introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated idene is shown below.
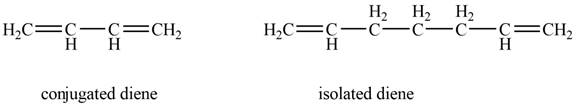
Figure 1
Answer to Problem 16.1P
The given diene is an isolated diene.
Explanation of Solution
Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The given diene is shown below.
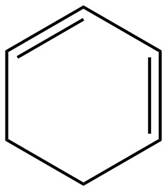
Figure 5
In the given diene, two double bonds are separated by more than single bond. Thus, the given diene is an isolated diene.
The given diene is an isolated diene.
Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY (LL) W/CONNECT
- Label each compound as aromatic, antiaromatic, or not aromatic. Assume all completely conjugated rings are planar. Å a. b. C. d.arrow_forward12.47 What diene and dienophile are needed to prepare each Diels-Alder product? a. b. free C. d. e. f. CNarrow_forwardDraw the product of each Diels-Alder reaction and indicate the stereochemistry at all stereogenic centers. A a. b.arrow_forward
- Draw the products formed in each reaction sequence. a. b. CI [1] Ph3P Br [2] BuLi H [3] [1] Ph3P [2] BuLi [3] Harrow_forwardProblem 13.28 What reagent is needed to convert (CH3)2CHCH₂ COCI to each compound? a. b. C. d. но Ho ГОНarrow_forwardAnswer the following questions about compounds A–D.a.How are the compounds in each pair related? Choose from constitutional isomers, stereoisomers, or identical molecules: A and B; A and C; B and D. b.Label each compound as a cis or trans isomer. c.Draw B as a hexagon with wedges and dashed wedges to show the stereochemistry of substituents. d.Draw a stereoisomer of A as a hexagon using wedges and dashed wedges to show the orientation of substituents.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





