
Concept explainers
Interpretation:
The IR band in Figure 16-33 that indicates an
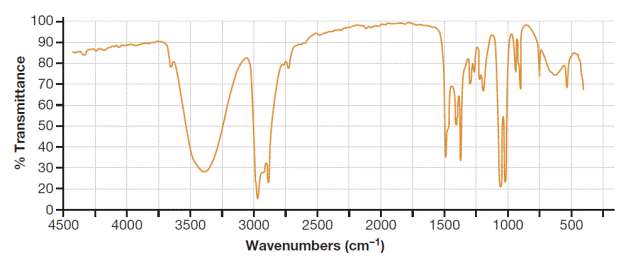

Concept introduction:
In general, the
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
ORGANIC CHEMISTRY SMARTWORK5 - ACCESS
- 5. Suppose that the CH3 group connected to the nitrogen in methamphetamine were instead a CH2CH3 group. Briefly describe how the spectrum would change, including the number of signals, the splitting patterns, and integration. Ignore everything that's the same, I'm just looking for the differences. CH2: CH3: 6. Consider the structure of the drug Adderall, shown to the right. Briefly, what differences would you see in the spectrum of Adderall versus the spectrum of methamphetamine? NH3 Part 2: Mechanismsarrow_forwardA compound with a molecular formula of C5 H100 results in the following IR spectrum. What functional groups are most likely? Select all correct answers. Infrared Spectrum 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 3500 3000 2500 2000 1500 1000 Wavenumbers (cm-1) alcohol alkene ketone ether Transmitancearrow_forwardCan someone help me identify the functional groups in this infrared spectrum? I know that I'm suppost to look between the 4000-1500cm^-1 frequency range. Is there ONLY a CH alkane functional group in this compound? Or is that very small peak in the C=O at 1700 count as one? Cuz isn't the C=O suppost to be the biggest peak?arrow_forward
- The DEPT-90 spectrum exhibits 6 in the 0-50 ppm region The DEPT-135 spectrum exhibits x 100 ppm region that is a positive ▾ C6 signal(s) for the CH groups: ▼ 1,2,6 ✓ in the sp2 hybridized region 100-150 C3 and C4 ▼ signal(s) (only the quaternary carbon atoms, signal(s), indicating the presence of a methylene group (CH₂) attached to an oxygen atom, are missing); there is C5 ▼ C1 and C2 ▼ and signal(s) in the 50-arrow_forwardQ1: Consider the two spectra shown below. One is for 3-methyl-1-cyclopentanone and the other one is for 3-methyl-1-cyclohexanone. Identify which spectra belongs to one compound explain your reasoning. Identify the important absorption peaks correspond to a functional group. Describe the absorption peaks. am 100 4900 400 3000 d 1500 1300 REG ho DEFE 18 PES SAFE RECERES 100arrow_forward500 20 8.0 Interpret the 'H-NMR spectrum and assign the correct structure. Please be sure to explain your work. C₂H₁, CL 3.0 7.0 400 4.0 6.0 5.0 -B00 5.0 6.0 4.0 200 7.0 E 3.0 8.0 MA 20 100 9.0 1.0 10.0 (ppm) 가 O Hz 08 (ppm)arrow_forward
- Consider a molecule with the molecular formula of C3H7NO2. The IR and ¹H NMR spectra are shown below. Draw the structure that best fits this data. 4000 3000 2H 2000 1500 Wavenumbers (cm-¹) + 2H 1000 3H 500 ppmarrow_forward100. 80- 60- 40- 20- 0. 4000 3500 3000 2500 2000 1500 1000 Wavenumber (cm) What information is available in the IR spectrum shown? A) It has a functional group of alcohol B) It has a functional group of amine C) It has a crboxylic group D) It has a benzene ring % Transmittancearrow_forwardBelow are three MS spectra, Spectra 1, 2, and 3. Each of this mass spectrum corresponds to either Compound A (contains one Cl in the molecular formula), Compound B (one I in the molecular formula), and Compound C (one Br in the molecular formula). Identify which spectra corresponds to which compound. Provide explanations for your choices.arrow_forward
- Compound CsH12 gives the following H-NMR. Draw the structure of the compound. Draw a box around the structure you want graded. 5.00 1.00 6.04 70 6.5 6.0 4.0 3.5 3.0 25 20 1.5 A student was adding bromine across the double bond of 2-butene to make 2,3-dibromobutane. After taking the NMR, the student discovered they didn't get the product expected. Based on the NMR, what product did they obtain? Draw a box around your answer. 1.00 2.01 |2.00 3.00arrow_forward4- A compound with the general formula C4H6 and having two types of signals in the HNMR spectrum is 2-Butyne. Q/ true or false 5- The compound C4H9Br that has only one signal in the NMR spectrum is the compound tert-butyl bromide 6- The proton of the aldehyde group appears at a low field because the movement of electrons in the C=O bond is generated An inscribed magnetic field opposite to the external applied magnetic field.arrow_forwardYou have an unknown with an absorption at 1680 cm-1; it might be an amide, an isolated double bond, a conjugated ketone, aconjugated aldehyde, or a conjugated carboxylic acid. Describe what spectral characteristics you would look for to help youdetermine which of these possible functional groups might be causing the 1680 peak.arrow_forward
