
Concept explainers
Interpretation: “The condensed structural formula and IUPAC name of two structural isomers of carboxylic acid having molecular formula
Concept introduction:
- Isomers: Compounds which have the same molecular formula, but different physical or chemical properties are known as isomers and the phenomenon is known as isomerism.
- Structural isomerism: The isomers with same molecular formula but different structural formula or different arrangement of atoms in a molecule are known as structural isomers.
- Condensed structural formula: The complete structural formula can be abbreviated by omitting some or all the covalent bonds and by indicating the number of identical groups attached to an atom by a subscript. This is known as condensed structural formula.
- IUPAC: The term IUPAC is International union of pure and applied chemistry. It provides the set of rules for assigning names to organic compounds.
- Rules for determining the IUPAC names
- The longest continuous chain of carbon atom is taken as the parent chain on which the various alkyl groups are considered side chain.
- Numbering is done from that side where functional group is near.
- If more than one functional group is present, then preference is given to that functional group which is senior in the seniority table group
If a side chain occurs more than once, the numerical prefixes di(for two),tri(for three) etc are added before the names of the group.
When there are two identical side chains at one position, numbers are given for each.
To draw and explain: The condensed structural formula, IUPAC name of the isomers of carboxylic acid having molecular formula
Solutions:
The condensed structural formula and IUPAC name of the structural isomers of carboxylic acid having molecular formula
Explanation of Solution
The carboxylic acid having molecular formula
Let us first draw the two structural isomers of carboxylic acid having molecular formula
a) 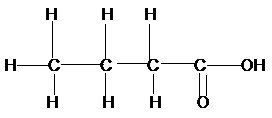
b) 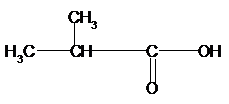
Now for (a), if we count the
Again for (b), if we count the
Therefore, the condensed structural formula, IUPAC name of the structural isomers of carboxylic acid having formula
a)
b)
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, and Biological Chemistry: Structures of Life, Books a la Carte Edition (5th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





