
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
8th Edition
ISBN: 9781305582453
Author: Brown
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.50P
(R)-Pulegone is converted to (R)-citronellic acid by addition of HCl followed by treatment with NaOH.
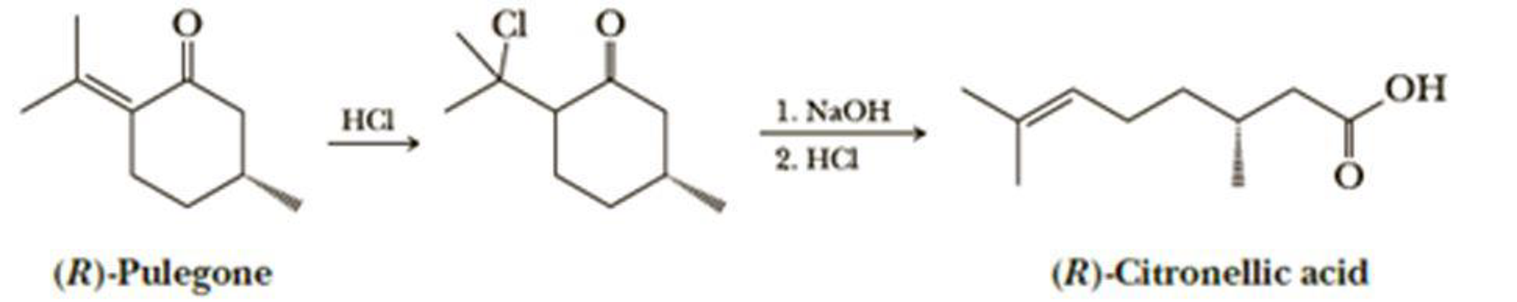
Propose a mechanism for each step in this transformation and account for the regioselectivity of HCl addition.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Devise a synthesis of the ketone hexan-3-one, CH3CH2COCH2CH2CH3, from CH3CH2Br as the only organic starting material; that is, all the carbon atoms in hexan-3-one must come from CH3CH2Br. You may use any other neededreagents.
When cis-2-decalone is dissolved in ether containing a trace of HCI, an equilibrium is
established with trans-2-decalone. The latter ketone predominates in the equilibrium
mixture.
H
H
HCI
cis-2-Decalone
trans-2-Decalone
Propose a mechanism for this isomerization and account for the fact that the trans iso-
mer predominates at equilibrium.
- PL 2-butanol slowly racemizes to give (R/S)-2-butanol in dilute sulfuric acid. Propose a mechanism to
account for this observation.
Chapter 16 Solutions
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
Ch. 16.1 - Write the IUPAC name for each compound. Specify...Ch. 16.1 - Write structural formulas for all aldehydes with...Ch. 16.1 - Write the IUPAC name for each compound.Ch. 16.5 - Prob. 16.4PCh. 16.6 - Prob. 16.5PCh. 16.7 - Prob. 16.6PCh. 16.7 - Write a mechanism for the acid-catalyzed...Ch. 16.8 - Prob. 16.8PCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - The given mechanism of transamination reaction is...
Ch. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - Prob. DQCh. 16.8 - Prob. EQCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.9 - Predict the position of the following equilibrium.Ch. 16.9 - Draw a structural formula for the keto form of...Ch. 16.10 - Prob. 16.11PCh. 16.11 - What aldehyde or ketone gives these alcohols upon...Ch. 16.11 - Prob. 16.13PCh. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - The infrared spectrum of compound A, C6H12O, shows...Ch. 16 - Following are 1H-NMR spectra for compounds B...Ch. 16 - Draw structural formulas for the product formed by...Ch. 16 - Suggest a synthesis for the following alcohols...Ch. 16 - Show how to synthesize the following alcohol using...Ch. 16 - 1-Phenyl-2-butanol is used in perfumery. Show how...Ch. 16 - Prob. 16.22PCh. 16 - Draw structural formulas for (1) the...Ch. 16 - Show how to bring about the following conversions...Ch. 16 - Prob. 16.25PCh. 16 - Wittig reactions with the following -chloroethers...Ch. 16 - Prob. 16.27PCh. 16 - Prob. 16.28PCh. 16 - 5-Hydroxyhexanal forms a six-membered cyclic...Ch. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - Propose a mechanism to account for the formation...Ch. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - Show how to bring about the following conversion.Ch. 16 - A primary or secondary alcohol can be protected by...Ch. 16 - Prob. 16.37PCh. 16 - Prob. 16.38PCh. 16 - Prob. 16.39PCh. 16 - Prob. 16.40PCh. 16 - The following molecule belongs to a class of...Ch. 16 - When cis-2-decalone is dissolved in ether...Ch. 16 - Prob. 16.43PCh. 16 - Prob. 16.44PCh. 16 - The following bicyclic ketone has two -carbons and...Ch. 16 - Propose a mechanism for this reaction.Ch. 16 - The base-promoted rearrangement of an -haloketone...Ch. 16 - If the Favorskii rearrangement of...Ch. 16 - (R)-Pulegone, readily available from pennyroyal...Ch. 16 - (R)-Pulegone is converted to (R)-citronellic acid...Ch. 16 - Starting with cyclohexanone, show how to prepare...Ch. 16 - Show how to convert cyclopentanone to these...Ch. 16 - Prob. 16.53PCh. 16 - Prob. 16.54PCh. 16 - Prob. 16.55PCh. 16 - Following is the structural formula of Surfynol, a...Ch. 16 - Prob. 16.57PCh. 16 - Propose a mechanism for this isomerization.Ch. 16 - Starting with acetylene and 1-bromobutane as the...Ch. 16 - Prob. 16.60PCh. 16 - Prob. 16.61PCh. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - All rearrangements we have discussed so far have...Ch. 16 - In dilute aqueous base, (R)-glyceraldehyde is...Ch. 16 - Treatment of -D-glucose with methanol in the...Ch. 16 - Treating a Grignard reagent with carbon dioxide...Ch. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Write the products of the following sequences of...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Prob. 16.78PCh. 16 - Prob. 16.79PCh. 16 - Prob. 16.80PCh. 16 - Prob. 16.81P
Additional Science Textbook Solutions
Find more solutions based on key concepts
The method to determine the volume of a powered solid, liquid and a rock needs to be determined. Concept introd...
Living by Chemistry
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach (4th Edition)
The active ingredient in Tylenol and a host of other over-the-counter pain relievers is acetaminophen (C8H9NO2)...
Chemistry: Atoms First
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
General, Organic, and Biological Chemistry - 4th edition
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ethylene oxide is the starting material for the synthesis of 1,4-dioxane. Propose a mechanism for each step in this synthesis.arrow_forwardDihydropyran is synthesized by treating tetrahydrofurfuryl alcohol with an arenesulfonic acid, ArSO3H. Propose a mechanism for this conversion.arrow_forwardA step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forward
- Using your reaction roadmap as a guide, show how to convert cyclohexanol into racemic trans-1,2-cydohexanediol. Show all required reagents and all molecules synthesized along the way.arrow_forwardAcid-catalyzed hydrolysis of the following epoxide gives a trans diol. Of the two possible trans diols, only one is formed. How do you account for this stereoselectivity?arrow_forwardTreatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.arrow_forward
- Claisen rearrangement of an allyl phenyl ether with substituent groups in both ortho positions leads to the formation of a para-substituted product. Propose a mechanism for the following rearrangement.arrow_forwardCyclohexene can be converted to 1-cyclopentenecarbaldehyde by the following series of reactions. Propose a structural formula for each intermediate compound.arrow_forwardThe formation of Br2 from NBS first involves the reaction of NBS with HBr to form an iminol intermediate and molecular bromine. The intermediate then undergoes acid-catalyzed tautomerism to form succinimide, the byproduct of the reaction. Propose a curved-arrow mechanism for the conversion of NBS into succinimide that also accounts for the formation of Br2.arrow_forward
- Propose a detailed mechanism for the following reactions: a) Reaction of 3-methylbutanal and diethyl malonate in the presence of sodium ethoxide solution in ethanol. b) Reaction of diethyl hexanedioate in the presence of sodium ethoxide followed by acid work up to form a beta-ketoester.arrow_forwardThe following questions concern ethyl (2-oxocyclohexane)carboxylate.(a) Write a chemical equation showing how you could prepare ethyl (2-oxocyclohexane)-carboxylate by a Dieckmann cyclization.(b) Write a chemical equation showing how you could prepare ethyl (2-oxocyclohexane)-carboxylate by acylation of a ketone.(c) Write structural formulas for the two most stable enol forms of ethyl (2-oxocyclohexane)carboxylate.(d) Write the three most stable resonance contributors to the most stable enolate derived from ethyl (2-oxocyclohexane)carboxylate.(e) Show how you could use ethyl (2-oxocyclohexane)carboxylate to prepare 2-methylcyclohexanone.(f) Give the structure of the product formed on treatment of ethyl (2-oxocyclohexane)-carboxylate with acrolein (H2C=CHCH=O) in ethanol in the presence of sodium ethoxidearrow_forwardIdentify A, B, and C, intermediates in the synthesis of the five-membered ring called an α- methylene-γ-butyrolactone. This heterocyclic ring system is present in some antitumor agents.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License