
Concept explainers
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
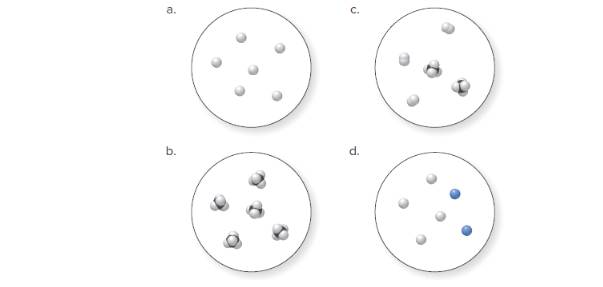
(a)
Interpretation:
Whether the given molecular art is a pure compound, mixture, or a pure element needs to be classified.
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 19P
Molecular art 'a' − Pure element.
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now, based on these definitions, molecular art (a) signifies pure elements.
(b)
Interpretation:
Whether the below molecular art is pure compound, mixture, or a pure element needs to be determined.
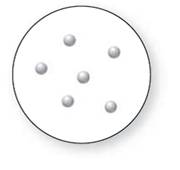
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 19P
Molecular art 'b' − Pure compounds
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now based on these definitions, molecular art (b) signifies pure compounds.
(c)
Interpretation:
Whether the below compound is a pure compound, mixture, or a pure element needs to be determined.
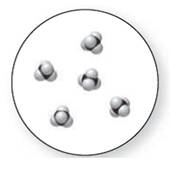
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 19P
Molecular art 'c' − Mixture
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now based on these definitions, molecular art (c) signifies mixture.
(c)
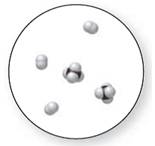
Interpretation:
Whether the below compound is a pure compound, mixture, or a pure element needs to be determined.
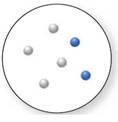
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 19P
Mixture
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now, based on these definitions, molecular art (d) signifies mixture.
Want to see more full solutions like this?
Chapter 1 Solutions
General, Organic, and Biological Chemistry - 4th edition
Additional Science Textbook Solutions
Chemistry
Chemistry & Chemical Reactivity
EBK INTRODUCTION TO CHEMISTRY
Organic Chemistry (9th Edition)
- If iron filings are placed with excess powdered sulfur in a beaker, the iron filings are still attracted by a magnet and could be separated from the sulfur with the magnet. Would this combination of iron and sulfur represent amixtureor apure substance?arrow_forwardHow does an element differ from a compound? How are they similar?arrow_forwardClassifying Matter Determine whether each of the following is a pure element, a compound, or a mixture. If it is a mixture, classify it as homogeneous or heterogeneous: a. pure salt b. helium gas c. chicken noodle soup d. coffeearrow_forward
- Which of the following are elements, and which are compounds? a NaOH; b BaCl2; c He; d Ag; e Fe2O3.arrow_forwardClassify each of the following as an element, a compound, or a mixture: (a) copper (b) water (c) nitrogen (d) sulfur (e) air (f) sucrose (g) a substance composed of molecules each of which contains two iodine atoms (h) gasolinearrow_forwardWhich of the following particulate illustrations represent pure substances and which represent mixtures?arrow_forward
- Diamonds and graphite are two forms of carbon. Carbon is an element. Chunks of graphite are sprinkled among the diamonds on a jewelers display tray. Is the material on the tray a pure substance or a mixture? Is the display homogenous or heterogeneous? Justify both answers.arrow_forwardA cup of coffee is an example of: a. a liquid pure substance b. a gaseous mixture c. a solid pure substance d. a liquid mixture e. a solid mixturearrow_forwardClassify each of the following pure substances as either an element or a compound: a C; b C2H5OH; c Cl2.arrow_forward
- 1f a piece of hard, white blackboard chalk is heated strongly in a flame, the mass of the piece of chalk will decrease, and eventually the chalk will crumble into a white dust. Does this change suggest that the chalk is composed of an element or a compound?arrow_forwardWhich of the following contains an element, a compound, and a mixture? copper, silicon dioxide(SiO2) , copper(II) sulfate(CuSO4) hydrogen, carbon dioxide(CO2) , water(H2O) chili, pizza, steak sodium, sodium chloride (NaCl). salt water nitrogen, argon, airarrow_forward1.74 What are the two properties of ITO that make it serve its function in touch screen applications?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





