
Concept explainers
(a)
Interpretation:
Whether erythrulose contains an aldehyde or ketone should be identified.
Concept Introduction:
Compounds which contain carbonyl carbon bonded to hydrogen or carbon atom will be considered as
Aldehydes − at least one H atom bonded to the carbonyl group.
Ketones − has two alkyl groups bonded to the carbonyl group.
Answer to Problem 16.55P
Erythrulose contains a ketone group.
Explanation of Solution
Given:
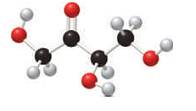
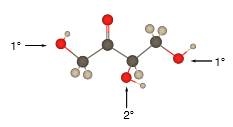
In the stick and ball structure, C atoms are representing in black color, H atoms in white color and O atoms in red color.
Stick and ball structure can be simplified as below;
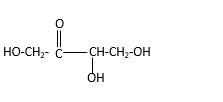
In erythrulose there is a carbonyl group that connects to two alkyl groups, thus, the erythrulose contains ketone.
(b)
Interpretation:
Hydroxyl groups in erythrulose should be classified as
Concept Introduction:
Alcohol- is an organic compound contains the group of
Answer to Problem 16.55P
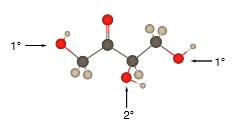
Explanation of Solution
In the below stick and ball structure, C atoms are representing in black color, H atoms in white color and O atoms in red color.
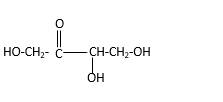
Stick and ball structure can be simplified as below;
Bonds that are indicated in red color are the
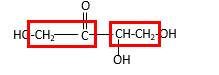
Bond that is indicated in blue color is the one that binds to two carbon atoms which makes an
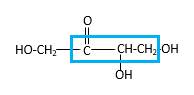
Therefore, OH groups in erythrulose can be assigned as below.
(c)
Interpretation:
The products should be identified by the reaction of erythrulose with Tollens reagent.
Concept Introduction:
Silver oxide in aqueous ammonium hydroxide reagent (
Answer to Problem 16.55P
No reaction.
Explanation of Solution
Addition of an O atom into a molecule is known as oxidation. If a carbonyl atom consists of a hydrogen atom directly connected to the carbonyl C, it will be oxidized in the presence of an oxidizing agent such as

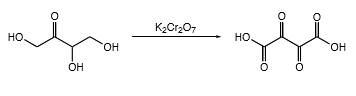
(d)
Interpretation:
The products should be identified by the reaction of erythrulose with
Concept Introduction:
Addition of an O atom into a molecule is known as oxidation. If a carbonyl atom consists of a hydrogen atom directly connected to the carbonyl C, it will be oxidized in the presence of an oxidizing agent such as
But, OH groups will be oxidized into respective carboxylic acids, aldehydes or ketones.
Answer to Problem 16.55P
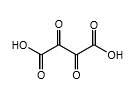
Explanation of Solution
In erythrulose molecule there are two primary alcohol groups and those groups will end up with a carboxylic acid and one secondary alcohol group in the erythrulose molecule will end up with a ketone by oxidation with
Want to see more full solutions like this?
Chapter 16 Solutions
GENERAL ORGANIC+BIOCHEM (LL)W/CONNECT
- Draw a structure for a lactone with 5 carbonsarrow_forward1. Draw the structure of 1-linolenyl-2-arachidyl-3-phosphatidylserine 2. Draw the structure of testosterone, the primary male sex hormone, which has a ketogroup at carbon 3 instead of hydroxyl group, the double bond at carbon 4 instead atcarbon 5, and a hydroxyl group instead of the alkyl group at carbon 17arrow_forwardConvert the following compound to a cyclic beta sugararrow_forward
- Draw the products formed when each alkene is treated with HCl.arrow_forwardGlycerol contains: a. oxygens which are each bonded to two alkyl groups b. oxygens single-bonded to primary and secondary carbons c. Oxygens double-bonded to carbon, with alkyls on both sides d. Oxygens double-bonded to carbon, with alkyls on one side only e. Oxygens double-bonded to carbon, with an alkyl on one side and an --OH on the other sidearrow_forward3-Methyl-2-hexenoic acid (mixture of E and Z isomers) has been identified as the substance responsible for the odor of human sweat. Synthesize the compound from raw materials that have five carbons or less.arrow_forward
- Amino acids such as glycine are the building blocks of large molecules called proteins that give structure to muscle, tendon, hair, and nails. What product is formed when glycine is treated with concentrated HCl? What product is formed when glycine is treated with NaOH?arrow_forwardDraw the products formed when (CH3)2C=CH2 is treated with following reagent. [1] BH3; [2] H2O2, HO−arrow_forwardstructure of each type of compound. A. D –aldotriose B. L-ketohexosearrow_forward
- Which of the following statements is INCORRECT? The carbon atom in formic acid is sp2 hybridized. Carboxylic acids with 5 or fewer carbons are soluble in water. Carboxylic acids are weaker acids than HCl. Carboxylic acids dissociate completely in water.arrow_forwardWhat product is formed when the compound is treated with Tollens reagent, (Ag2O, NH4OH)?arrow_forwardAlkene is converted to alkyl halide by reaction with HCl . (T/F) Iodoform is used as an antiseptic. (T/F) Intermolecular bonding is strongest in alcohols than phenols. (T/F) Formation of blood red colour in Victor Meyer’s test indicated the presence of primary alcohol. (T/F) Fructose is a pentahydroxyketone . (T/F)arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

