
Concept explainers
(a)
Interpretation: The product formed by the reaction of given diene with one equivalent of
Concept introduction: Diene is a hydrocarbon that contains two
Answer to Problem 16.60P
The products formed by the given reaction are,
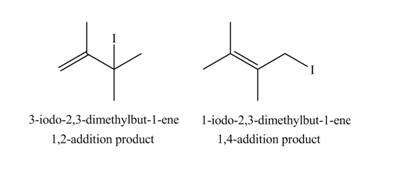
Explanation of Solution
Conjugated dienes undergoes electrophilic addition to gives a mixture of products that is
Markovnikov addition of
The given reaction is shown below.
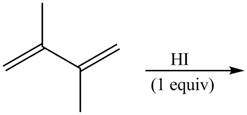
Figure 1
The given diene is a conjugated diene. The attack of
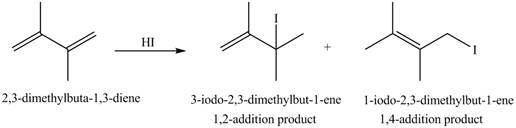
Figure 2
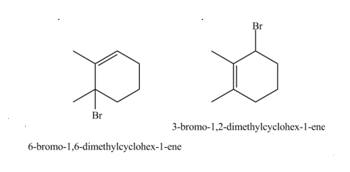
Therefore, the products formed by the given reaction are
The products formed by the given reaction is
(b)
Interpretation: The Diels-Alder product formed by the given reaction including stereochemistry is to be drawn.
Concept introduction: A
Answer to Problem 16.60P
The Diels-Alder product formed by the given reaction is,
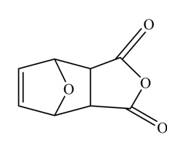
Explanation of Solution
The given reaction is shown below.
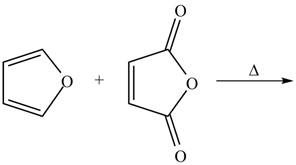
Figure 3
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. They have syn stereochemistry. The stereochemistry of dienophile in endo isomer is same as that of bridging ring system, whereas in exo isomer, it is in opposite.
Thus, the Diels-Alder product formed by the given reaction including stereochemistry is shown in Figure 4.
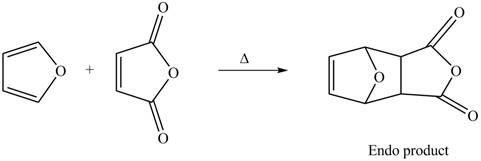
Figure 4
The Diels-Alder product formed by the given reaction including stereochemistry is shown in Figure 4.
(c)
Interpretation: The Diels-Alder product formed by the given reaction including stereochemistry is to be drawn.
Concept introduction: A chemical reaction that involves
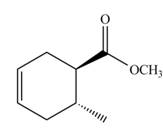
Answer to Problem 16.60P
The Diels-Alder product formed by the given reaction including stereochemistry is,
Explanation of Solution
The given reaction is shown below.
![]()
Figure 5
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. They have syn stereochemistry.
Thus, the Diels-Alder product formed by the given reaction including stereochemistry is shown in Figure 4.
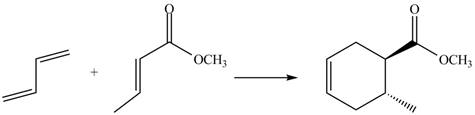
Figure 6
The Diels-Alder product formed by the given reaction including stereochemistry is shown in Figure 6.
(d)
Interpretation: The product formed by the reaction of given diene with one equivalent of
Concept introduction: Diene is a hydrocarbon that contains two
Answer to Problem 16.60P
The products formed by the given reaction are,
Explanation of Solution
Conjugated dienes undergoes electrophilic addition to gives a mixture of products that is
Markovnikov addition of
The given reaction is shown below.
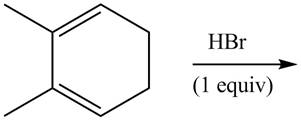
Figure 7
The given diene is a conjugated diene. The attack of
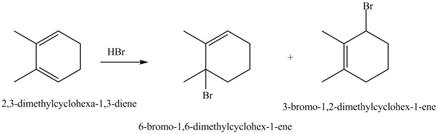
Figure 8
Therefore, the products formed by the given reaction is
The products formed by the given reaction is
Want to see more full solutions like this?
Chapter 16 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
- Addition of HCl to alkene X forms two alkyl halides Y and Z. (A) Label Y and Z as a kinetic or thermodynamic product and explain why. (B) Explain why the addition of HCl occurs at the exocyclic C=C, rather than the other C=Carrow_forwardDraw the product (A) of the following Diels–Alder reaction. A was a key intermediate in the synthesis of the addicting pain reliever morphine, the chapter-opening molecule.arrow_forwardhelp ! a. What is the relationship between (+)-neomenthol and (-)-menthol? b. Assign absolute stereochemistry (r/s) to each of the stereocenters in (+)-menthol and (-)-menthol (DRAW) c.. Draw the structures of all of menthol's stereoisomerz .. THanks..arrow_forward
- Sulfur ylides, like the phosphorus ylides, are usefulintermediates in organic synthesis. Methyl trans-chrysanthemate, anintermediate in the synthesis of the insecticide pyrethrin I,can be prepared from diene A and a sulfur ylide. Draw a stepwisemechanism for this reaction.arrow_forwardWhich is an energy diagram for a concerted reaction (SN2 and E2)? A B C Darrow_forwardDevise a stepwise synthesis of attached compound from dicyclopentadieneusing a Diels–Alder reaction as one step. You may also use organiccompounds having ≤ 4 C's, and any required organic or inorganicreagents.arrow_forward
- Dienynes undergo metathesis to afford fused bicyclic ring systems. (a) Explain how A is converted to B. (b) Keeping this reaction in mind, draw the two products formed by dienyne metathesis of C.arrow_forwarddraw the structure and include stereochemistry.arrow_forwardDeconstruct the given Diels–Alder adduct. Draw the reactants (A and B), in any order, that would be needed to produce the Diels–Alder adduct.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
