
Interpretation:
The structure of the starting alcohol is to be determined, and a complete, detailed mechanism to account for each of the products based on the
Concept introduction:
Alcohols undergo a substitution reaction when treated with a strong Bronsted acid such as hydrobromic acid. Hydroxyl group is not a good leaving group but can be made into one by protonation of the oxygen atom in it as a first step. In the second step, the bromide ion serves as a nucleophile and attacks the carbon attached to the protonated hydroxyl group. Thus, a bromine is attached to the carbon atom, which was originally attached to the hydroxyl group, and the substitution occurs.
In
In addition to chemical shift, a
Complicated splitting patterns can result when a proton is unequally coupled to two or more protons that are different from one another.
The ideal range for
The integration of each signal suggests the number of protons responsible for that signal. The splitting pattern of a signal indicates the number of neighboring protons that are distinct from the protons responsible for that signal. To deduce the structure of an unknown compound, the first step is to find the index of hydrogen deficiency if the molecular formula is given. Based on the data given in the
Answer to Problem 16.88P
The structure of the starting alcohol that produces a mixture of
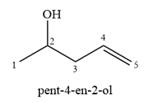
A complete, detailed mechanism is
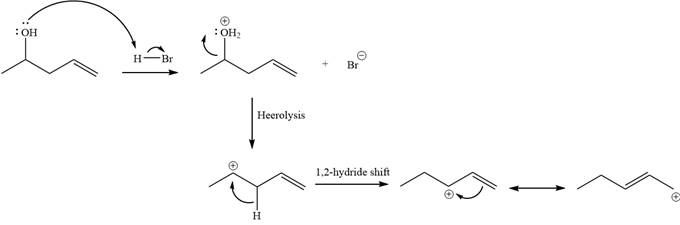
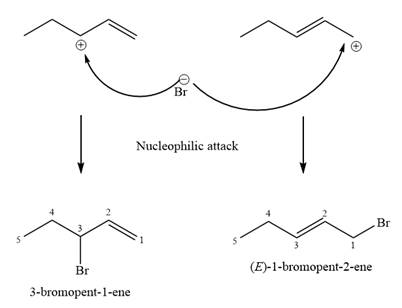
Explanation of Solution
The given reaction is
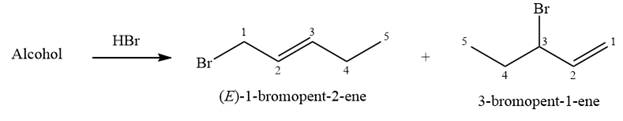
Alcohols undergo a substitution reaction when treated with a strong Bronsted acid such as hydrobromic acid. Hydroxyl group is not a good leaving group but can be made into one by protonation of the oxygen atom in it as a first step. In the second step, the bromide ion serves as a nucleophile and attacks the carbon attached to the protonated hydroxyl group. Thus, a bromine is attached to the carbon atom which was originally attached to the hydroxyl group, and the substitution occurs. Thus, the retrosynthetic analysis would look like
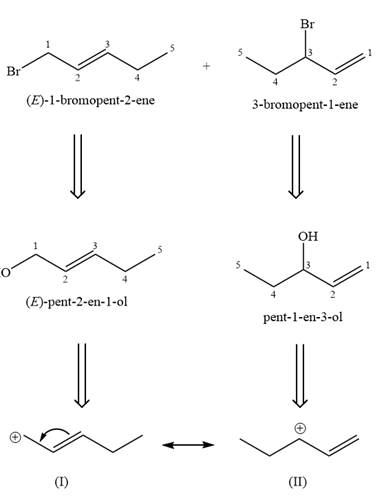
The two given products can be imagined to come from those two allyllic carbocations. The two carbocations are resonance structures. There can be three possible allylic alcohols responsible for the formation of these carbocations.
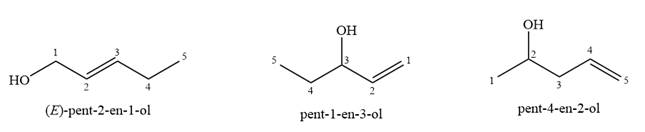
Given
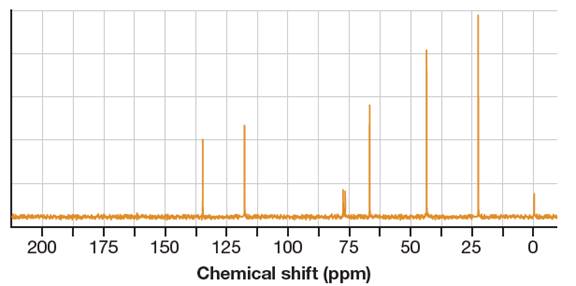
It shows six distinct signals indicating six distinct carbon atoms in the starting alcohol.
This NMR does not reveal the identity of the starting alcohol as all the possible alcohols have five distinct carbon atoms.
Let’s see the
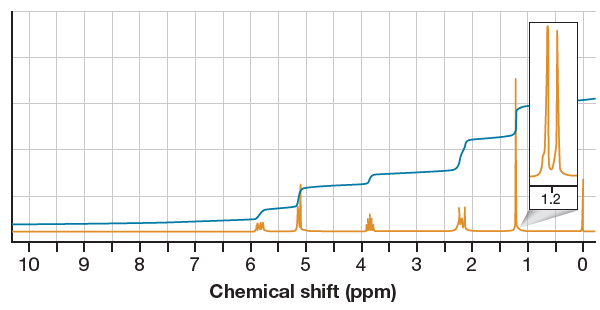
In the given
Two signals at
The peak
Step 1
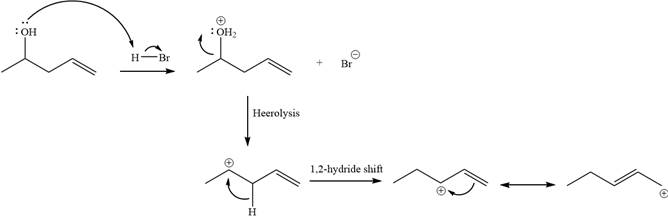
Step 2:
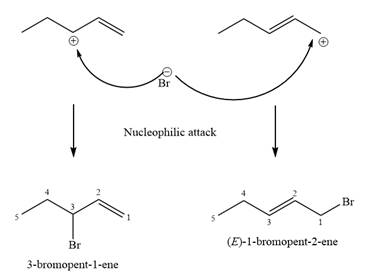
The structure of the unknown starting alcohol is proposed based on its
Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY:PRINCIPLES...(CL)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





