
(a)
Interpretation:
The IUPAC name for the ester formed when acetic acid and ethanol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
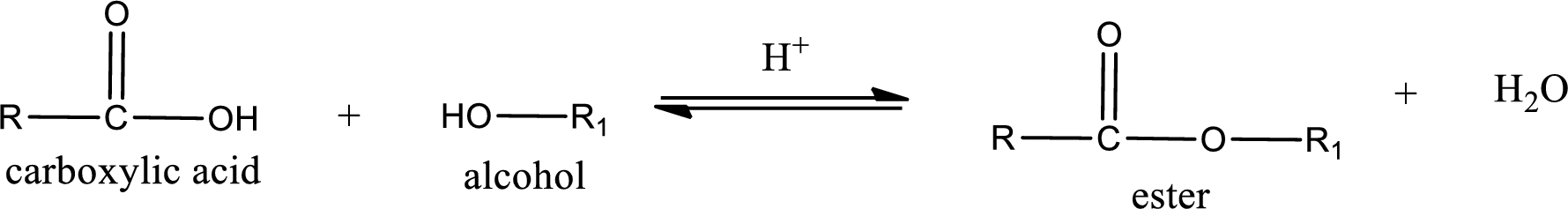
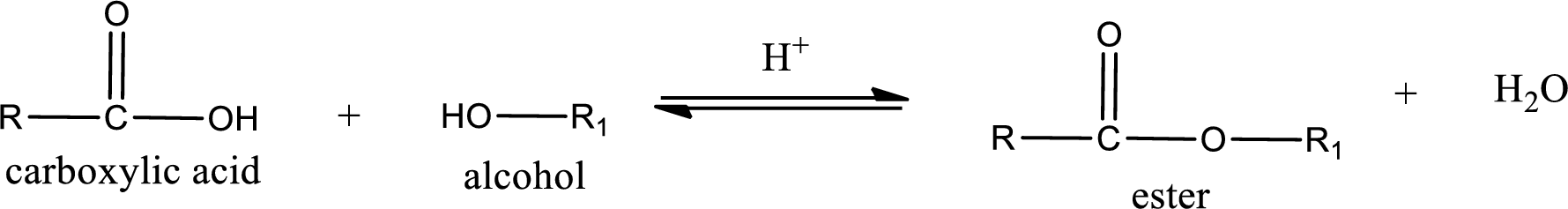
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
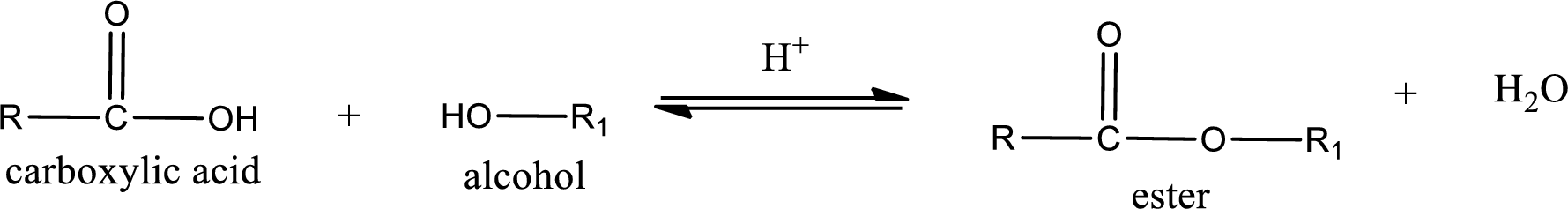
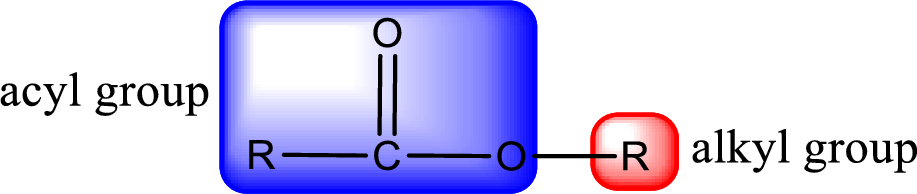
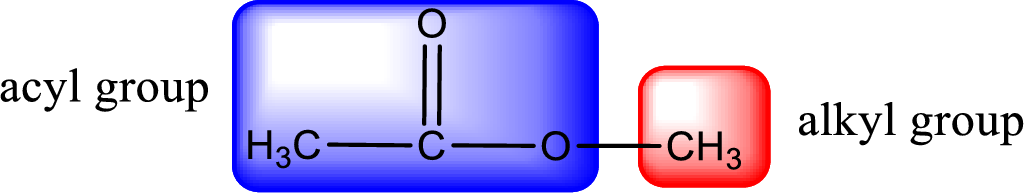
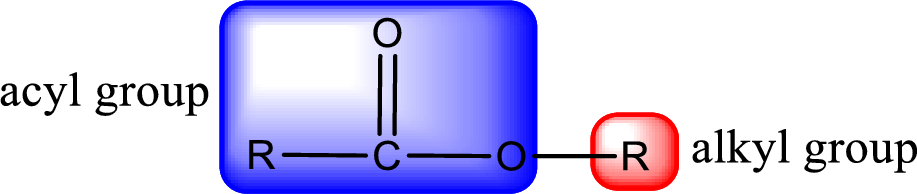
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
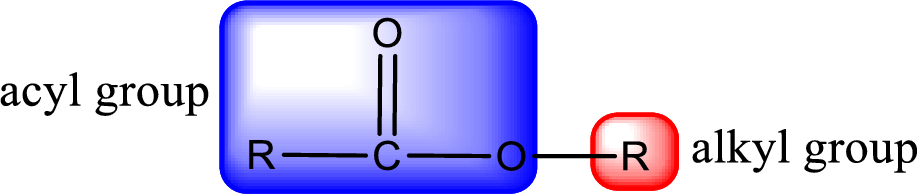
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(a)
Answer to Problem 16.97EP
IUPAC name of the ester formed is ethyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
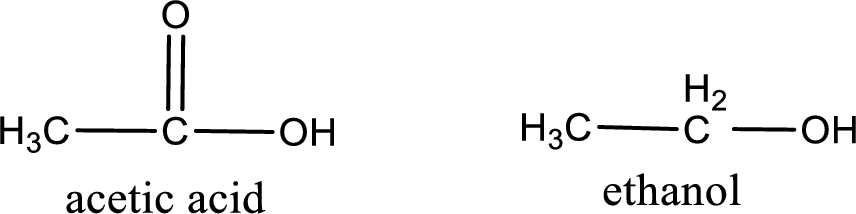
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,
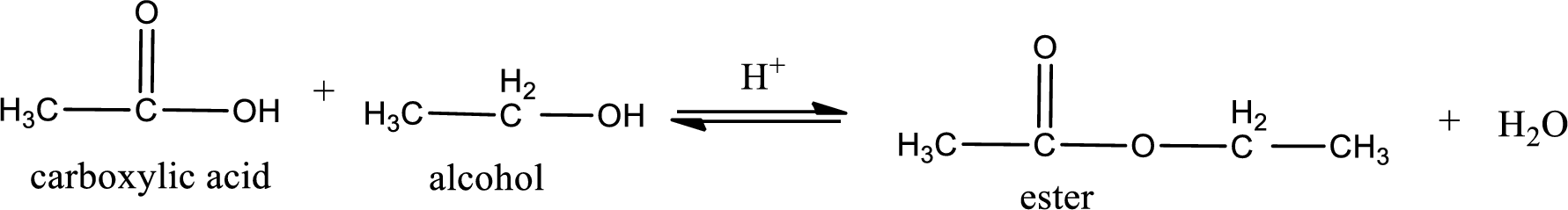
The structure of ester is,
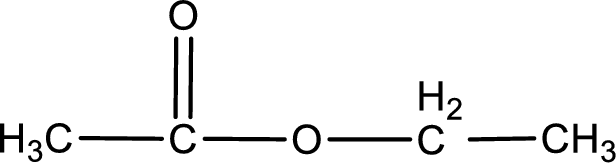
The alkyl and acyl group is identified as shown below,
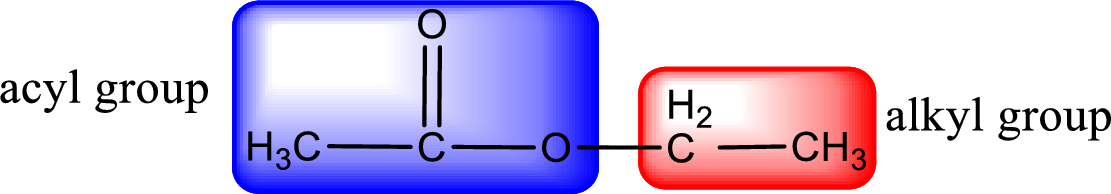
Alkyl group contains only two carbon atoms. Hence alkyl group is named as ethyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is ethyl ethanoate.
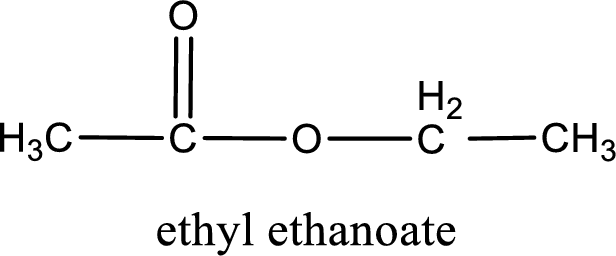
IUPAC name of the formed ester is assigned.
(b)
Interpretation:
The IUPAC name for the ester formed when ethanoic acid and methanol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
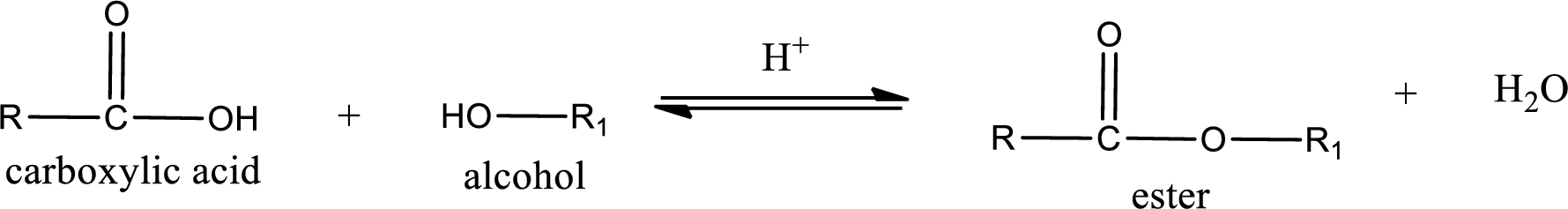
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(b)
Answer to Problem 16.97EP
IUPAC name of the ester formed is methyl ethanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
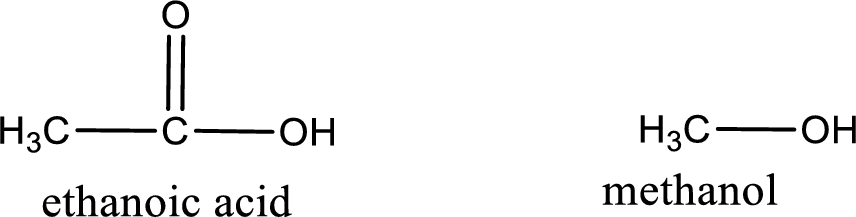
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,
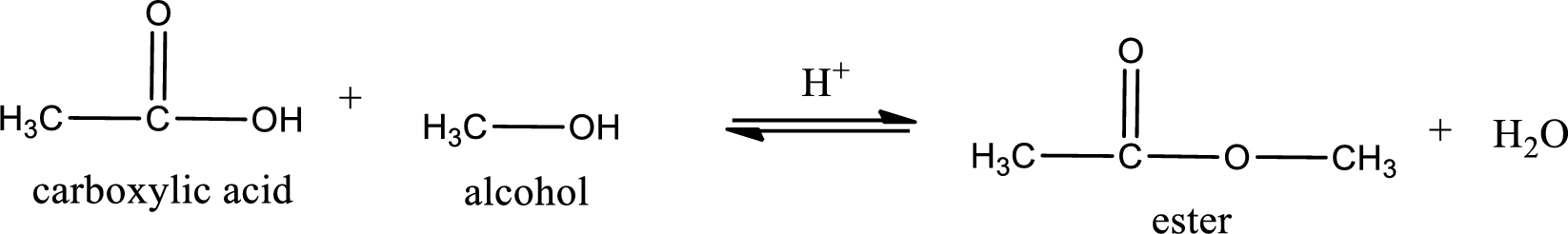
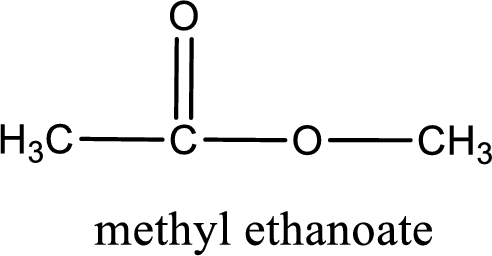
The structure of ester is,
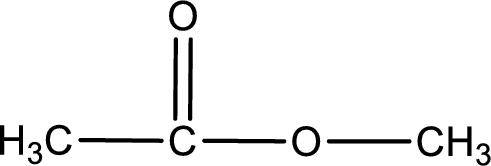
The alkyl and acyl group is identified as shown below,
Alkyl group contains only one carbon atom. Hence alkyl group is named as methyl. The acyl group contains two carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is ethanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as ethanoate. Therefore IUPAC name of the given ester is methyl ethanoate.
IUPAC name of the formed ester is assigned.
(c)
Interpretation:
The IUPAC name for the ester formed when butyric acid and ethyl alcohol react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(c)
Answer to Problem 16.97EP
IUPAC name of the ester formed is ethyl butanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
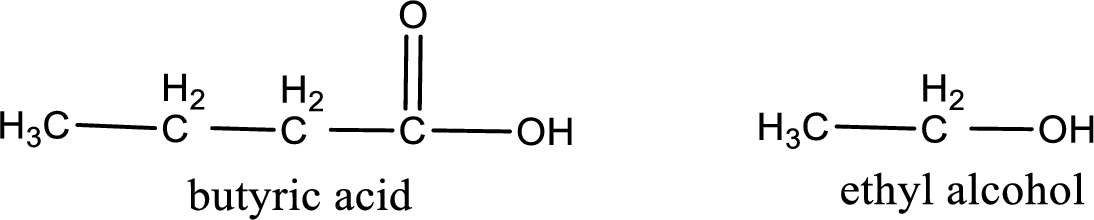
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,

The structure of ester is,
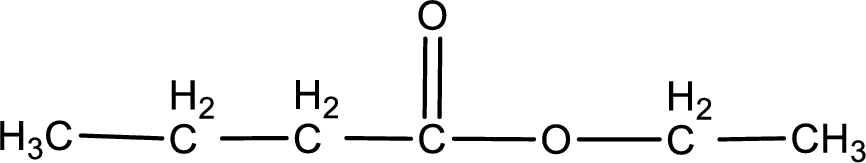
The alkyl and acyl group is identified as shown below,
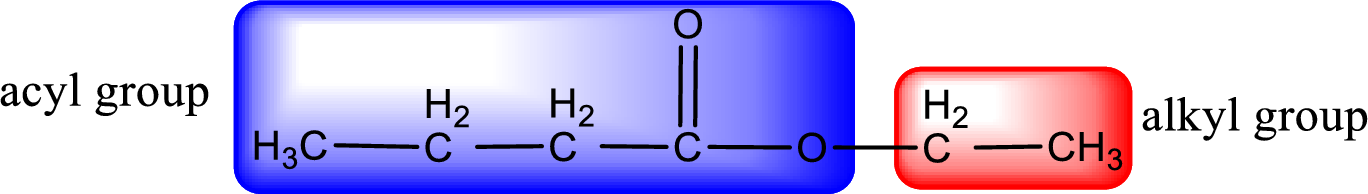
Alkyl group contains only two carbon atoms. Hence alkyl group is named as ethyl. The acyl group contains four carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is butanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as butanoate. Therefore IUPAC name of the given ester is ethyl butanoate.
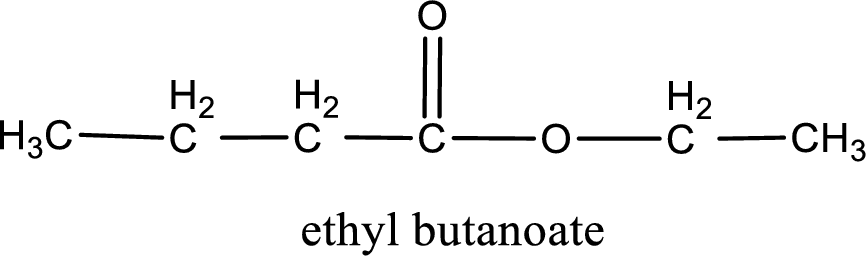
IUPAC name of the formed ester is assigned.
(d)
Interpretation:
The IUPAC name for the ester formed when 2-butanol and caproic acid react has to be assigned.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
For naming an ester, it can be structurally viewed in a way that contains an acyl group and an alkyl group.
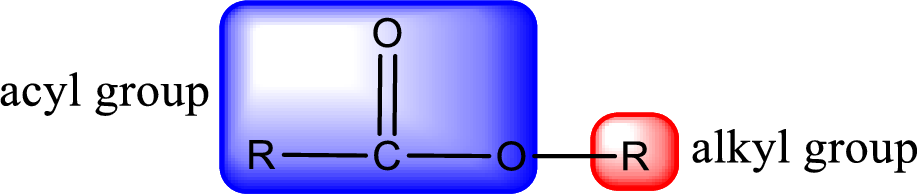
Rules to obtain IUPAC name and common name for an ester:
- Alkyl part appears first in the IUPAC name and it is followed by the acyl part of ester as a separate word.
- Name of the alkyl part in the ester is just a name of R group. It can be alkyl, cycloalkyl, or aryl group.
- Acyl part present in the ester is named by considering the acid name and replacing the suffix “-ic acid” with “-ate”.
- To obtain the common name the alkyl part name is the same while the acyl part name is derived from the common name of the acid by replacing the suffix “-ic acid” with “-ate”.
(d)
Answer to Problem 16.97EP
IUPAC name of the ester formed is 1-methylpropyl hexanoate.
Explanation of Solution
Given carboxylic acid and alcohol structure is,
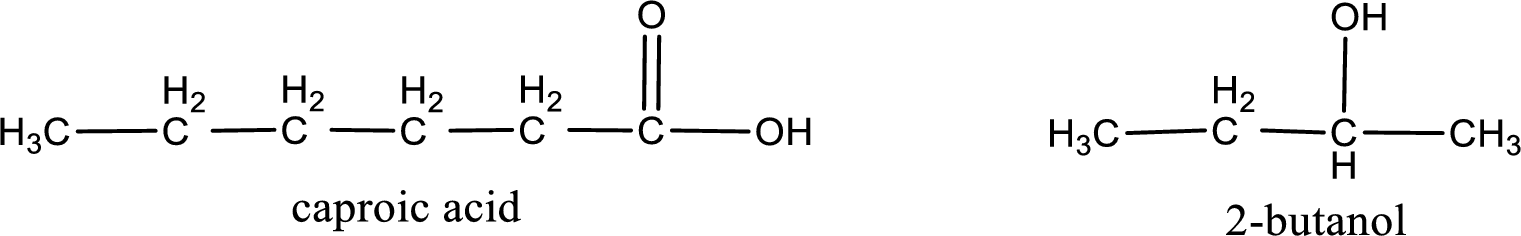
The reaction between two compounds that are shown above, result in the formation of ester. The structure of the ester formed and the complete reaction can be given as shown below,

The structure of ester is,
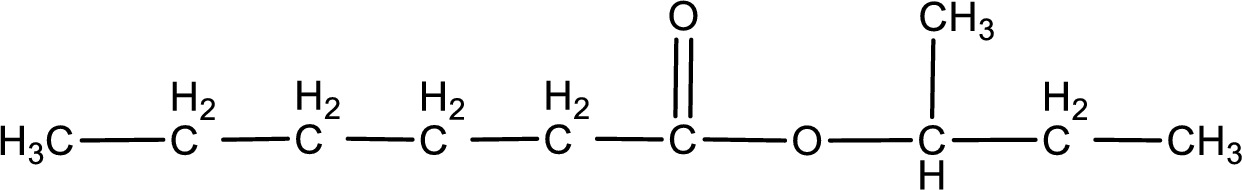
The alkyl and acyl group is identified as shown below,
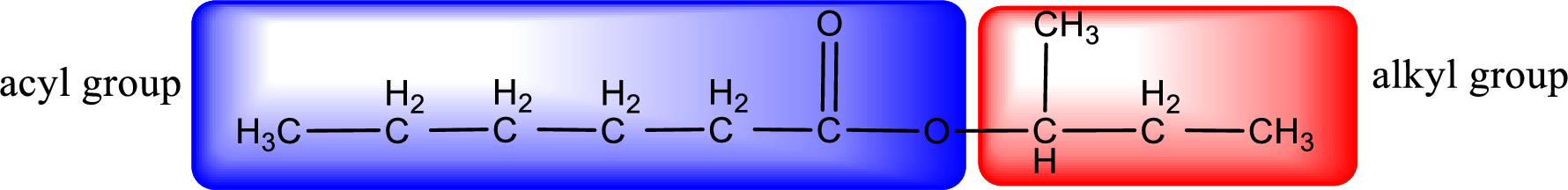
Alkyl group contains a total of four carbon atoms. In that alkyl group (propyl) a methyl group is substituted in the first carbon atom. Hence alkyl group is named as 1-methylpropyl. The acyl group contains six carbon atoms. The IUPAC name of carboxylic acid that contains three carbon atoms is hexanoic acid. For naming acyl group in ester the suffix “-ic acid” is converted into “-ate”. This gives the name of acyl part as hexanoate. Therefore IUPAC name of the given ester is 1-methylpropyl hexanoate.
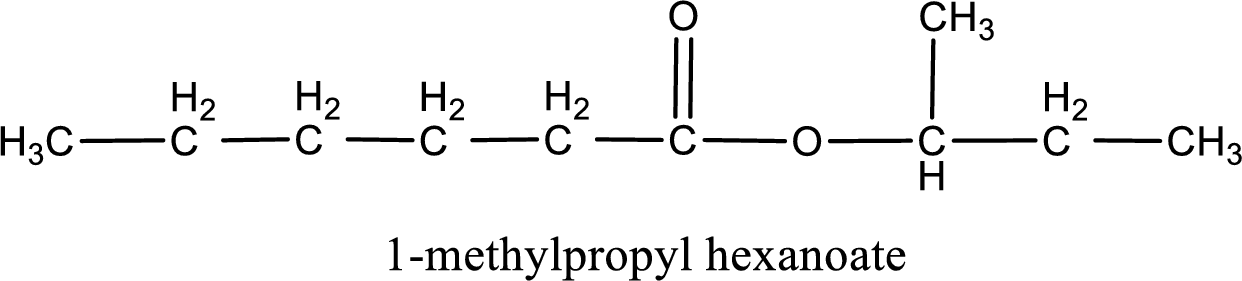
IUPAC name of the formed ester is assigned.
Want to see more full solutions like this?
Chapter 16 Solutions
General, Organic, And Biological Chemistry, Hybrid (with Owlv2 Quick Prep For General Chemistry Printed Access Card)
- Which of the following classifications apply to the formation of 3-hydroxybutyrate from acetoacetate?(a) Condensation (b) Hydrolysis(c) Oxidation (d) Reductionarrow_forwardThe components of poison ivy and poison oak that produce the characteristic itchy rash are catechols substituted with long-chain alkylgroups. If you were exposed to poison ivy, which of the treatments below would you apply to the affected area? Justify your choice.(a) Wash the area with cold water.(b) Wash the area with dilute vinegar or lemon juice.(c) Wash the area with soap and water.(d) Wash the area with soap, water, and baking soda (sodium bicarbonate).arrow_forwardWith respect to the parent compound, explain in detail how modifying the rings shown as A, B, C, D and E in morphine can affect the biological activity associated with the moleculearrow_forward
- What is fentanyl and why is the scope of the problem so deadly in the US?arrow_forwardExplain what indole alkaloids are. And describe the significance of vinflunine.arrow_forwardWrite the reaction, occurring in the liver, that causes theoxidation of ethanol. What is the product of this reactionand what symptoms are caused by the product?arrow_forward
- Define the following terms: a. alkylating agents b. base analogue c. nonalkylating agent d. intercalating agent e. ethidium bromidearrow_forwardHow can chirality and stereoisomers influence the pharmacology, bioactivity, toxicology, pharmacokinetics, and metabolism of ibuprofen? Please provide a detailed summary of what might happen if, for instance, the R enantiomer isn't able to be inverted to the bioactive S enantiomer.arrow_forwardDraw the structures of the following acids:(a) 2-Ethyl-3-hydroxyhexanoic acid (b) m-Nitrobenzoic acidarrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





