
Concept explainers
Write the structure of the principal organic product formed in the reaction of
Sulfuric acid (catalytic amount), heat at
°C
Sulfuric acid (catalytic amount), heat at
°C
Dimethyl sulfoxide (DMSO), oxalyl chloride
Pyridinium chlorochromate (PCC) in dichloromethane
Potassium dichromate
Acetic acid 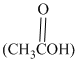 in the presence of dissolved hydrogen chloride
in the presence of dissolved hydrogen chloride
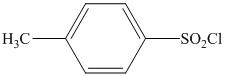 in the presence of pyridine
in the presence of pyridine
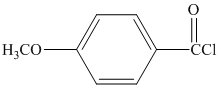 in the presence of pyridine
in the presence of pyridine
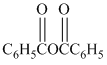 in the presence of pyridine
in the presence of pyridine
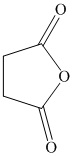 in presence of pyridine
in presence of pyridine
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
CAREY: ORGANIC CHEMISTRY
- Write structural formulas for all ketones with the molecular formula C6H12O and give each its IUPAC name. Which of these ketones are chiral?arrow_forwardArrange these compounds in order of increasing acidity: 2,4-dichlorophenol, phenol, cyclohexanol.arrow_forwardThree products with the molecular formula C6 H4BrCl form when bromobenzene is treated with chlorine, Cl2, in the presence of FeCl3 as a catalyst. Name and draw a structural formula for each product.arrow_forward
- Draw a structural formula for the major organic product of the following reaction: CH2Cl2, + Br2arrow_forwardCompound A and compound B are different alcohols with the same molecular formula of C4H10O. When reacted with chromic acid (H2CrO4), compound A and compound B produce compound C and compound D, respectively. Compound C has a molecular formula of C4H8O2. Compound E is obtained when compound A reacts with pyridinium chlorochromate (PCC) in a solvent such as dichloromethane (CH2Cl2). Draw the structure of compound A, B, C, D and E.arrow_forwardDiethyl ether[(CH3CH2)2O]is an imperfect anesthetic in part because it readily combusts in air to form CO2 and H2O. Write a balanced equation for the complete combustion of diethyl ether.arrow_forward
- Write the reagent or draw structures of the starting material or organic product(s) in the following reactions. If more than one product is formed, identify the major product where possible. (a) (b) HO OH OH H2SO4 ? Cl₂ ? FeCl3arrow_forwardEsterication is the reaction of a carboxylic acid (RCOOH) with an alcohol (R'OH) to form an ester (RCOOR') with loss of water. Equation [1] is an example of an intermolecular esterication reaction. Equation [2] is an example of an intramolecular esterication reaction; that is, the carboxylic acid and alcohol are contained in the same starting material, forming a cyclic ester as product. The equilibrium constants for both reactions are given. Explain why Keq is different for these two apparently similar reactions.arrow_forwardAldehydes and ketones react with one molecule of an alcohol to form compounds called hemiacetals, in which there is one hydroxyl group and one ether-like group. Reaction of a hemiacetal with a second molecule of alcohol gives an acetal and a molecule of water. We study this reaction in Chapter 16. Draw structural formulas for the hemiacetal and acetal formed from these reagents. The stoichiometry of each reaction is given in the problem.arrow_forward
- Give IUPAC names for the following substances:arrow_forward17-60 1-Propanol can be prepared by the reduction of an aldehyde, but it cannot be prepared by the acid catalyzed hydration of an alkene. Explain why it cannot be prepared from an alkene.arrow_forwardAccount for the fact that treating propenoic acid (acrylic acid) with HCl gives only 3-chloropropanoic acid.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole




