
Concept explainers
dentify each of the following
NMR spectra:
Interpretation:
The structures of each of the
Concept introduction:
In
The number of signals in a spectrum gives information about the type of carbon atoms present in the structure of the compound.
In
The
Index of hydrogen deficiency (IHD) is calculated by the equation as follows:
Here,
Oxygen atoms do not disturb the index of hydrogen deficiency.
At
At
A methine is
Answer to Problem 38P
Solution: The structures of each of the
a)
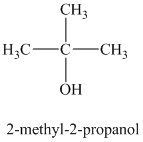
b)
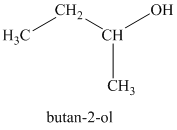
c)
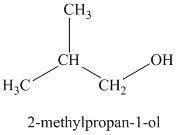
Explanation of Solution
a)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the alcohol contains no ring or multiple bonds. The
The signal
The signal
The structure must contain three equivalent methyl groups and one tertiary carbon atom. Therefore, the structure of this isomer is shown as follows:
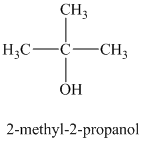
This isomer is a tertiary alcohol.
b)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the alcohol contains no ring or multiple bonds. The
The signal
The signal
The signal
Thus, the structure must contain two non-equivalent methyl groups, one methylene group, and one methine group to which the hydroxyl group is attached.
Therefore, the structure of this isomer is shown as follows:

This isomer is a secondary alcohol.
c)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the alcohol contains no ring or multiple bonds. The
The signal
The signal
The signal
Thus, the structure must contain two equivalent methyl groups, one methylene group, and one methine group to which the hydroxyl group is attached.
Therefore, the structure of this isomer is shown as follows:

Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- What is the structure of the compound with the formula C10H14O, if it shows a strong broad IR signal near 3300 cm-1, the 1H-NMR spectrum is: 1.44 ppm (9H singlet) 4.97 ppm (1H broad singlet) 6.90 ppm (2H doublet) 7.32 ppm (2H doublet)arrow_forwardHow many unique carbon atoms are in hexane (CH3CH2CH2CH2CH2CH3)? In other words, how many peaks would you expect in its 13C NMR spectrum? 1 2 3 6arrow_forwardIdentify the structures of isomers H and I (molecular formula C8H11N).a.Compound H: IR absorptions at 3365, 3284, 3026, 2932, 1603, and 1497 cm−1b.Compound I: IR absorptions at 3367, 3286, 3027, 2962, 1604, and 1492 cm−1arrow_forward
- How many peaks would you expect to see in the 13C NMR spectrum of 3-bromohexane? a.) 3 b.) 4 c.) 5 d.) 6arrow_forward4 Which of these C6H14 isomers has the greatest number of ¹3C NMR signals? (a) Hexane (d) 2,2- (b) Dimethylbutane 2-Methylpentane X3-Methylpentane (e) 2.3- Dimethylbutanearrow_forwardWhat is the structure of the compound if the mass spectrum shows M+ = 72, IR shows peak near 1720 cm-1, 13C-NMR shows 4 lines. The proton NMR is: 2.4 ppm (2H quartet) 2.1 ppm (3H singlet) 1.1 ppm (3H triplet)arrow_forward
- What is the structure of the compound with the formula C5H12O, if it has a strong broad IR signal centered near 3330 cm-2, and the 1H-NMR spectrum is: 0.91 ppm (3H triplet) 1.19 ppm (6H singlet) 1.50 ppm (2H quartet) 2.24 ppm (1H singlet)arrow_forwardNMR. Indicate how many signals appear in the proton spectrum of the(1) CICH=CH2(2) o-chloromethylbenzenearrow_forward2) Compound C gave the following proton NMR signals: a) singlet,1.22 ppm, 6H; b) Triplet, 1.85 ppm, 2H, J = 7 Hz; c) triplet, 2.83 ppm, 2H, J = 7 Hz; d) singlet, 7.02 ppm, 4H. Sketch the proton NMR spectrum of compound C and assign the chemical shifts to the protons of the compounds.arrow_forward
- 1. There are several isomeric alkanes of molecular formula C6H14.Two of these exhibit the following 1H-NMR spectra. Propose a structure for each of the isomers. Isomer A: δ = 0.84 (d, 12 H), 1.39 (septet, 2H) ppm Isomer B: δ = 0.84 (t, 3 H), 0.86 (s, 9H), 1.22 (q, 2H) ppmarrow_forwardPropose a structural formula for each compound consistent with its 1H-NMR and 13C-NMR spectra. (a) C5H10O2 (b) C7H14O2 (c) C6 H12O2 (d) C7H12O4 (e) C4H7ClO2 (f) C4H6O2arrow_forward3-Bromo-1-phenyl-1-propene shows a complex NMR spectrum in which the vinylic proton at C2 is coupled with both the C1 vinylic proton (J = 16 Hz) and the C3 methylene protons (J = 8 Hz). Draw a tree diagram for the C2 proton signal, and account for the fact that a five-line multiplet is observed.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

