
Concept explainers
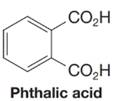
Compounds W and X are isomers; they have the molecular formula
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Living By Chemistry: First Edition Textbook
Chemistry: Structure and Properties
Living by Chemistry
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Organic Chemistry As a Second Language: Second Semester Topics
Organic Chemistry (8th Edition)
- Addition of m-xylene to the strongly acidic solvent HF/SbF5 at 45C gives a new species, which shows 1H-NMR resonances at 2.88 (3H), 3.00 (3H), 4.67 (2H), 7.93 (1H), 7.83 (1H), and 8.68 (1H). Assign a structure to the species giving this spectrum.arrow_forwardNitriles, R–=C≡N, undergo a hydrolysis reaction when heated with aqueous acid. What is the structure of the compound produced by hydrolysis of propanenitrile, CH3CH2C≡N, if it has IR absorptions from 2500–3100 cm-1 and at 1710 cm-1, and has M+=74?arrow_forwardAn aromatic compound K, whose molecular formula is C8H11N, is examined in the laboratory to elucidate its structure. The following observations were made: A) Compound K is soluble in dilute hydrochloric acid but insoluble in sodium hydroxide solution. B) Treatment of compound K with excess potassium hydroxide and benzenesulfonyl chloride, C(6)H(5)SO(2)Cl, results in the formation of a heterogeneous mixture. The NMR spectrum of compound K is shown below. C) Compound K when treated with acetic anhydride[CH3-C(O)-O-C(O)-CH3], gives compound L, whose molecular formula is C(10)H(13)ON. Compound L is insoluble in dilute acid or dilute base at room temperature, heating compound L in dilute acid or base, however, regenerates compound K. D) When compound L is heated with a mixture of concentrated nitric acid and sulfuric acid, a single product, compound M, with the molecular formula C(10)H(12)O(3)N(2) is formed in excellent yields. On the basis of these observations draw the structures of…arrow_forward
- Compounds Y and Z are isomers with the molecular formula C10H12O. The IR spectrum of each compound shows a strong absorption band near 1710 cm−1 . The 1H NMR spectra of Y and Z are given below. Propose structures for Y and Z.arrow_forwardA solution of acetone [(CH3)2C=O] in ethanol (CH3CH2OH) in the presence of a trace of acid was allowed to stand for several days, and a new compound of molecular formula C7H16O2 was formed. The IR spectrum showed only one major peak in the functional group region around 3000 cm−1, and the 1H NMR spectrum is given here. What is the structure of the product?arrow_forwardCompound X has molecular formula C5H10. In the presence of a metal catalyst, compound X reacts with one equivalent of molecular hydrogen to yield 2-methylbutane. (1) Suggest three possible structures for compound X. (2) Hydroboration-oxidation of compound X yields a product with no chirality centers. Identify the structure of compound X.arrow_forward
- Hydrocarbon X has the formula C6H12.X reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form a product having 12 primary hydrogens.Treatment of X with ozone followed by zinc in aqueous acid gives a mixture two aldehydes.What is the structure of X?arrow_forwardWhen a compound with molecular formula C11H14O2 undergoes acid-catalyzed hydrolysis, one of the products that is isolated gives the following 1H NMR spectrum. Identify the compound.arrow_forward19. A compound with molecular formula C6H12O2 exhibits two singlets in its 1H NMR spectrum, at d 1.4 (9H) and d 2.0 (3H). Its IR spectrum shows a strong absorption band near 1740 cm-1. What is the structure for this compound? Show the correlation of the spectra with the molecular structure.arrow_forward
- The treatment of (CH3)2C=CHCH2Br with H2O forms B (molecular formula C5H10O) as one of the products. Determine the structure of B from its 1H NMR and IR spectra.arrow_forwardCompounds B and C are isomers with molecular formula C5H9BrO2. The 1H NMR spectrum of compounds B and C are shown below. The IR spectrum corresponding to compound B showed strong absorption bands at 1739, 1225, and 1158 cm-1, while the spectrum corresponding to compound C have strong bands at 1735, 1237, and 1182 cm-1. 1.Based on the information provided, determine the structure of compounds B and C. 2.Assign all peaks in 1H NMR spectrum of compounds B and C.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

