
Concept explainers
Differentiate between the shaded areas in Figure 16.24at temperatures T 1 and T 2 on the basis of the number ofcollisions per unit time that might occur with energyequal to or greater than the activation energy.
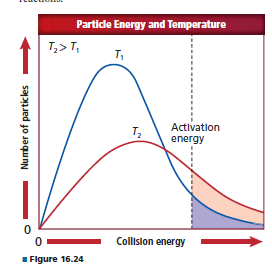
Interpretation:
The difference between the shaded areas in the figure at temperature
Concept introduction:
According to collision theory the number of collisions between reactant molecules is proportional to the rate of chemical reaction. At high temperature molecules gains energy therefore they collide faster.
Answer to Problem 84A
At high temperature particles are move faster so they have higher energy while at lower temperature particles are move slowly hence they have lower energy than the particles of higher energy.
Explanation of Solution
As shown in the figure,
From the graph it is clear that number of particles is higher at high temperature that means they have high collision energy than the lower temperature molecules.
Chapter 16 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Chemistry: A Molecular Approach (4th Edition)
Organic Chemistry (8th Edition)
General Chemistry: Principles and Modern Applications (11th Edition)
CHEMISTRY-TEXT
Organic Chemistry
Chemistry: The Central Science (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





