
THERMODYNAMICS-SI ED. EBOOK >I<
9th Edition
ISBN: 9781307573022
Author: CENGEL
Publisher: MCG/CREATE
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.6, Problem 77P
An ammonia–water absorption refrigeration unit operates its absorber at 0°C and its generator at 46°C. The vapor mixture in the generator and absorber is to have an ammonia mole fraction of 96 percent. Assuming ideal behavior, determine the operating pressure in the (a) generator and (b) absorber. Also determine the mole fraction of the ammonia in the (c) strong liquid mixture being pumped from the absorber and the (d) weak liquid solution being drained from the generator. The saturation pressure of ammonia at 0°C is 430.6 kPa, and at 46°C it is 1830.2 kPa.
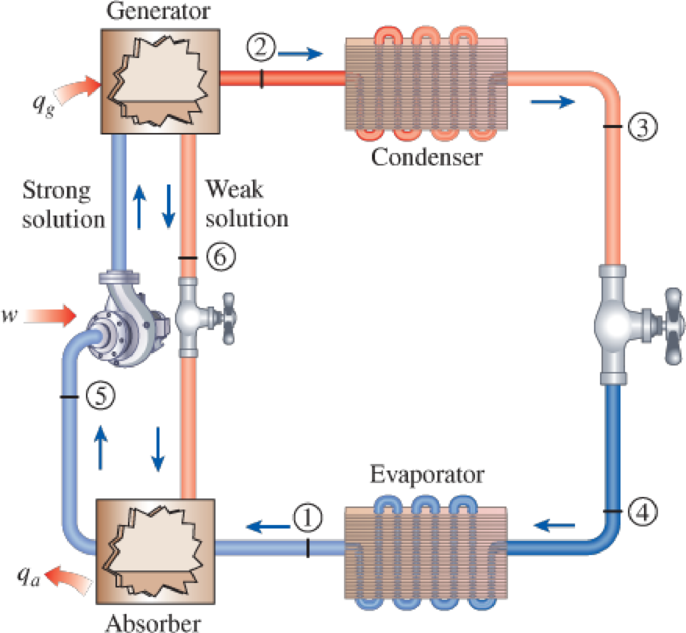
FIGURE P16–77
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An aqua ammonia absorption plant operates between pressures at 206.8 and 1378 kPaa. The strongsolution enters the generator saturated at 82.2°C and the weak solution leaves the generator saturated at93.3°C. Saturated vapor leaves the generator with a concentration of 0.993 kg NH3 /kg mixture. Assuming thatthe strong solution leaving the absorber is saturated, neglecting pump work and heat losses, calculate perkg/min flow of refrigerant through the evaporator:a. the mass flow of strong solution entering the generator in kg/minb. the mass flow of weak solution leaving the generator in kg/minc. the enthalpy of the weak solution entering the absorber in kJ/kg weak solution and the heat rejected in the absorber in kW
Saturated liquid at 1378 kPaa and 82.2°C has a concentration of 0.46 kg NH3 /kg mixture and an enthalpy of209.2 kJ/kg of mixture, saturated liquid at 1378 kPaa and 93.3°C has a concentration of 0.41 kg NH3 /kg mixtureand an enthalpy of 255.68 kJ/kg of mixture, saturated liquid…
The compression factor of a van deer Waals gas at 100°C and 100 atm given that a= 1.345 atm-L^2/mole^2 and b=0.0322 L/mole
Problem 13.053 Isobaric Heating
A mixture of nitrogen and carbon dioxide has a carbon dioxide mass fraction of 50 percent. This mixture is heated at constant pressure
in a closed system from 120 kPa and 30°C to 180°C. Calculate the work produced during this heating in kJ/kg. The universal gas
constant is Ru= 8.314 kJ/kmol-K. Use the table containing the molar mass, gas constant, and critical-point properties.
The work produced during this heating is
kJ/kg.
Chapter 16 Solutions
THERMODYNAMICS-SI ED. EBOOK >I<
Ch. 16.6 - Why is the criterion for chemical equilibrium...Ch. 16.6 - Write three different KPrelations for reacting...Ch. 16.6 - Is a wooden table in chemical equilibrium with the...Ch. 16.6 - A reaction chamber contains a mixture of CO2, CO,...Ch. 16.6 - A reaction chamber contains a mixture of N2and N...Ch. 16.6 - A reaction chamber contains a mixture of CO2, CO,...Ch. 16.6 - Which element is more likely to dissociate into...Ch. 16.6 - Prob. 8PCh. 16.6 - Prob. 9PCh. 16.6 - Prob. 10P
Ch. 16.6 - Prob. 11PCh. 16.6 - Prob. 12PCh. 16.6 - Prob. 13PCh. 16.6 - Prob. 14PCh. 16.6 - Prob. 15PCh. 16.6 - Prob. 16PCh. 16.6 - Prob. 17PCh. 16.6 - Prob. 18PCh. 16.6 - Prob. 19PCh. 16.6 - Prob. 20PCh. 16.6 - Prob. 21PCh. 16.6 - Prob. 22PCh. 16.6 - Prob. 23PCh. 16.6 - Determine the equilibrium constant KP for the...Ch. 16.6 - Prob. 26PCh. 16.6 - Prob. 27PCh. 16.6 - Carbon monoxide is burned with 100 percent excess...Ch. 16.6 - Prob. 30PCh. 16.6 - Prob. 31PCh. 16.6 - Estimate KP for the following equilibrium reaction...Ch. 16.6 - Prob. 33PCh. 16.6 - A mixture of 3 mol of N2, 1 mol of O2, and 0.1 mol...Ch. 16.6 - Prob. 35PCh. 16.6 - Prob. 36PCh. 16.6 - Prob. 37PCh. 16.6 - Prob. 38PCh. 16.6 - Prob. 40PCh. 16.6 - What is the equilibrium criterion for systems that...Ch. 16.6 - Prob. 43PCh. 16.6 - Prob. 44PCh. 16.6 - Prob. 45PCh. 16.6 - Prob. 47PCh. 16.6 - Prob. 48PCh. 16.6 - Prob. 51PCh. 16.6 - Prob. 52PCh. 16.6 - Prob. 53PCh. 16.6 - Prob. 54PCh. 16.6 - Prob. 55PCh. 16.6 - Prob. 56PCh. 16.6 - Prob. 58PCh. 16.6 - Prob. 59PCh. 16.6 - Prob. 60PCh. 16.6 - Prob. 61PCh. 16.6 - Using the Henrys constant data for a gas dissolved...Ch. 16.6 - Prob. 63PCh. 16.6 - Prob. 64PCh. 16.6 - Prob. 65PCh. 16.6 - Prob. 66PCh. 16.6 - A liquid-vapor mixture of refrigerant-134a is at...Ch. 16.6 - Prob. 68PCh. 16.6 - Prob. 69PCh. 16.6 - An oxygennitrogen mixture consists of 30 kg of...Ch. 16.6 - Prob. 71PCh. 16.6 - Prob. 72PCh. 16.6 - Prob. 73PCh. 16.6 - Prob. 74PCh. 16.6 - Prob. 75PCh. 16.6 - Prob. 76PCh. 16.6 - An ammoniawater absorption refrigeration unit...Ch. 16.6 - Prob. 78PCh. 16.6 - Prob. 79PCh. 16.6 - Prob. 80PCh. 16.6 - One lbmol of refrigerant-134a is mixed with 1...Ch. 16.6 - Prob. 82RPCh. 16.6 - Prob. 83RPCh. 16.6 - Prob. 84RPCh. 16.6 - Prob. 85RPCh. 16.6 - Prob. 88RPCh. 16.6 - Prob. 89RPCh. 16.6 - Prob. 90RPCh. 16.6 - Prob. 91RPCh. 16.6 - Prob. 92RPCh. 16.6 - A constant-volume tank contains a mixture of 1 mol...Ch. 16.6 - Prob. 94RPCh. 16.6 - Prob. 95RPCh. 16.6 - Prob. 96RPCh. 16.6 - Prob. 97RPCh. 16.6 - Prob. 99RPCh. 16.6 - Consider a glass of water in a room at 25C and 100...Ch. 16.6 - Prob. 101RPCh. 16.6 - Prob. 102RPCh. 16.6 - Prob. 105RPCh. 16.6 - Prob. 106RPCh. 16.6 - Prob. 107RPCh. 16.6 - Prob. 108RPCh. 16.6 - Prob. 109FEPCh. 16.6 - Prob. 110FEPCh. 16.6 - Prob. 111FEPCh. 16.6 - Prob. 112FEPCh. 16.6 - Prob. 113FEPCh. 16.6 - Prob. 114FEPCh. 16.6 - Propane C3H8 is burned with air, and the...Ch. 16.6 - Prob. 116FEPCh. 16.6 - Prob. 117FEPCh. 16.6 - The solubility of nitrogen gas in rubber at 25C is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- RP-1 is highly refined form of kerosene used for many first stage rocket engines. The average composition of it is indicated by CH1.9 a. What is the stoichiometric mixture ratio (MR) for RP-1 and oxygen? b. Now, you have a mixture of air and RP-1 with three times more air (in terms of moles) than is needed to burn all the fuel. How high is the final temperature? The heats of formation are given in the table below. In molar quantities, assume 1 mole of air is (O2+3.76N2). The reactants have a temperature of 25°C before combustion. You may use the average values of the specific heats for each constituent. C. Would the adiabatic flame temperature be lower or higher for a rocket engine that uses pure oxygen instead of air? Explain. Constituent Qf kJ/kmol @ 298 K Cp kJ/kmol K CH19 9,358 CO2 (g) -393,522 51.9 O2 (g) 0 34.0 N2 (g) 0 31.6 H₂0 (g) -241.827 40.6arrow_forwardAbsorption of Ammonia in a Tray Tower. A tray tower is to be used to remove 99% of the ammonia from an entering air stream containing 6 mol % ammonia at 293 K and 1.013 × 105 Pa. The entering pure water flow rate is 188 kg H₂O/h·m² and the inert air flow is 128 kg air/h- m². Calculate the number of theoretical trays needed. Use equilibrium data from Appendix A.3. For the dilute end of the tower, plot an expanded diagram to step off the number of trays more accurately.arrow_forwardProblem 13.033 Specific Heat and Molecular Weight The volumetric analysis of a mixture of gases is 30 percent oxygen, 40 percent nitrogen, 10 percent carbon dioxide, and 20 percent methane. Calculate the apparent specific heats and molecular weight of this mixture of gases. The universal gas constant is Ru = 8.314 kJ/kmol-K. Use the table containing the molar mass, gas constant, and critical-point properties and the table containing the ideal-gas specific heats of various common gases. The apparent molecular weight of this mixture of gases is The constant-pressure specific heat of the mixture is The constant-volume specific heat of the mixture is kg/kmol. kJ/kg-K. kJ/kg-K.arrow_forward
- 5. draw schematic for the problemarrow_forward1) A stream of outside air is mixed with a stream of return air in an air-conditioning system that operates at standard atmospheric pressure. The flow rate of outdoor air is 1/4 of the mixture flow rate and its condition is 32°C dry bulb temperature and 23°C wet bulb temperature. The flow rate of return air is 1/4 of the mixture flow rate and its condition is 24°C and 50% relativehumidity. Determine the following properties of the mixture: a) enthalpy b) specific humidityc dew paint temperature d) dry bulb temperature e) specific volumearrow_forward2 %0 l. Ii. . 7:1Y 5310126247118900636_2 h.w 1- Calculate the dryness fraction (quality) of steam which has 1.5 kg of water in suspension with 50 kg of steam. 2-A vessel having a volume of 0.6 m3 contains 3.0 kg of liquid water and water vapour mixture in equilibrium at a pressure of 0.5 MPa. Calculate (1) Mass and volume of liquid ; (2) Mass and volume of vapour 3-Determine the missing properties and the phase descriptions in the following table for water: T, °C Р, КРа u, kJ/kg Phase description (a) 200 0.6 (Ь) 125 1600 (c) 1000 2950 (d) 75 500 (e) 850 0.0 4-Determine the specific volume of superheated water vapor at 10 MPa and 400°C. 5-A 1.8-m3 rigid tank contains steam at 220°C. Onethird of the volume is in the liquid phase and the rest is in the vapor form. Determine (a) the pressure of the steam, (b) the quality of the saturated mixture. 6- Find the specific volume, enthalpy and internal energy of wet steam at 18 bar, dryness fraction 0.85. 7- Find the dryness fraction, specific…arrow_forward
- The moist air at 30 C° and 50 % RH enters steady state dehumidifier at the rate of 280 m³/min. The air passes over a cooling coil and water vapour condenses. The saturated moist air exists at 10 C°. The condensate also leaves the dehumidifier at 10 C°. The pressure is atmospheric. Determine 1- The mass flow rate of dry air. 2- The rate at which water is condensed. 3- The required refrigerating capacity in ton of refrigeration.arrow_forward9. Carbon Dioxide and Oxygen is placed each inside a 1m3 tank separated by an isolation valve. Initially, Carbon dioxide has the following properties: P = 20kPag & T = 25°C and that Oxygen has the following properties: P = 30kPag & T = 75°C. If heat is being prevented to escape in the outside surroundings, determine the resulting temperature of the mixture if it is found that after opening the isolation valve, the resulting pressure on both tank is 25kPag. Draw a figure or FBD that will support the problem. Explain each step by step formula.arrow_forwardThe following experiment is carried out: 10 moles of a gas are placed in a piston equipped with acylinder device so we can increase or reduce the pressure upon compression or expansion. Duringall the experimental steps, we will keep the experimental setup at a constant temperature of T =60°C. The initial pressure is 0.2 bar and the next steps are followed out:Step 1: The gas is compressed to P = 0.7 bar, where we see that condensation begins.Step 2: At the end of the condensation, the volume is 0.80 L. Equipment readings tell us that 495kJ of heat was removed during the condensation.Step 3: The liquid is then compressed to P = 13.5 bar and V = 0.77 L, where we see that solidsbegin to form.Step 4: At the end of the conversion of all the liquid into solid, the volume is 0.72 L and wemeasured 25 kJ of heat removed during this step. Use the above experimental observations to estimate each of the following properties for this compound. A) The isothermal compressibility of the liquid phaseB)…arrow_forward
- Which process results in the greatest endothermic change for 10.0g of H2Oarrow_forwardDefine each enthalpy obtained from tables and chart and draw the p-h diagramlabeling the respective evaporating and condensing pressures andtemperatures in each problem. Use the book, refrigeration and air conditioning 3rd edition hipolito b. sta. mariaarrow_forwardUsing the Clapeyron equation, determine the latent heat of vaporization of saturated Propane. Data: Temperature: 40°F; Pressure: 77.80 psia; Liquid volume: 0.03055 ft3/lbm; Vapor volume: 1.33 ft3/lbm.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning

Refrigeration and Air Conditioning Technology (Mi...
Mechanical Engineering
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:Cengage Learning
Chemical and Phase Equilibrium; Author: LearnChemE;https://www.youtube.com/watch?v=SWhZkU7e8yw;License: Standard Youtube License