
(a)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule is considered here.
The NMR signals for the
(a)
Explanation of Solution
Splitting in
In
In
.94ppm will the shift of
13.69ppm will the shift of
So the given values of the
Structural formula of the compound is;
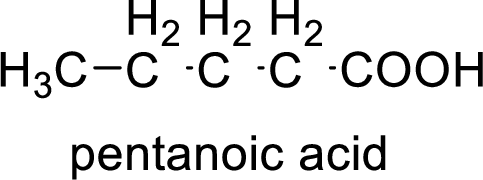
(b)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule is considered here.
The NMR signals for the
(b)
Explanation of Solution
Splitting in
In
In
1.08ppm will the shift of
30.69ppm will the shift of
So the given values of the
Structure formula of the compound is:
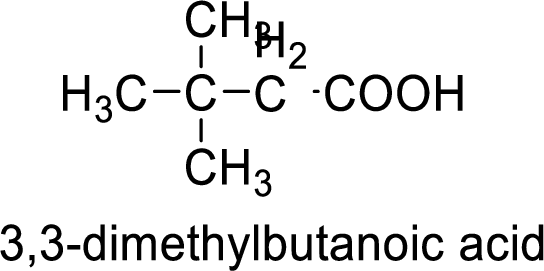
(c)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(c)
Explanation of Solution
Splitting in
In
In
.93ppm will the shift of
11.81ppm will be the shift of
So the given values of the
Structural formula of the product is;
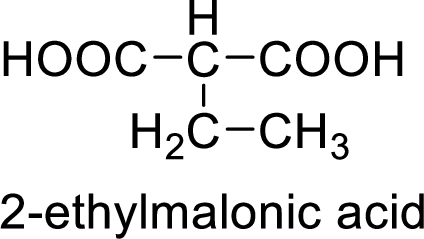
(d)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(d)
Explanation of Solution
Splitting in
In
In
1.29ppm will the shift of
22.56ppm will the shift of
So the given values of
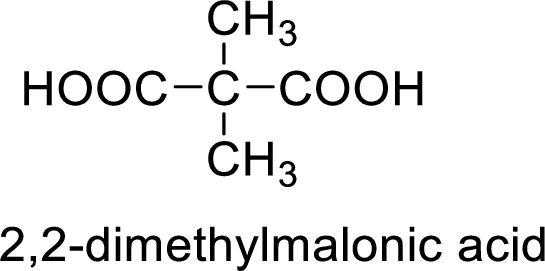
(e)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(e)
Explanation of Solution
Splitting in
In
In
1.91ppm will the shift of
18.11ppm will the shift of
So the given values of
Structural formula of the product;
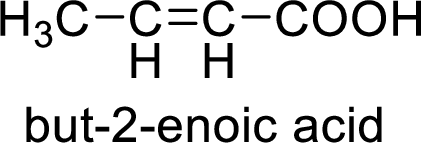
(f)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(f)
Explanation of Solution
Splitting in
In
In
2.34ppm will the shift of
34.02ppm will the shift of
So the given values of
Structure of the product
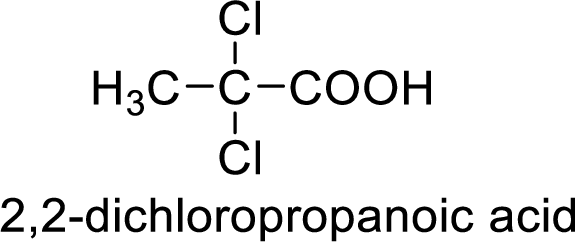
(g)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(g)
Explanation of Solution
Splitting in
In
In
1.42ppm will the shift of
13.69ppm will the shift of
So the given values of
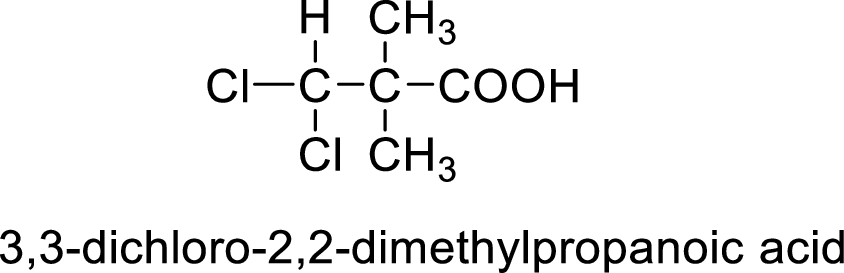
(h)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(h)
Explanation of Solution
Splitting in
In
In
97ppm will the shift of
13.24ppm will the shift of
So the given values of
Structural formula of the compound is;
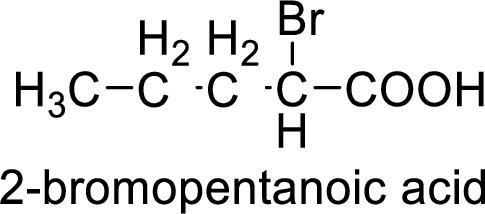
(i)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. A hydrogen nucleus with in the molecule is considered here.
The NMR signals for the
(i)
Explanation of Solution
Splitting in
In
In
3.38ppm will the shift of
34.75ppm will the shift of
So the given values of
So the structure of the product is;
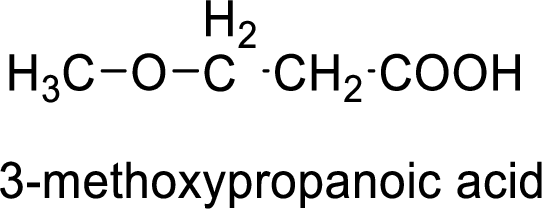
Want to see more full solutions like this?
Chapter 17 Solutions
ORG.CHEM:TXT+OWLV2+MINDTAP 6MTHS >BI<
- Compound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily in 10% Na2CO3 and 10% NaOH. When these alkaline solutions are acidified with 10% HCl, compound I is recovered unchanged. Given this information and its 1H-NMR spectrum, deduce the structure of compound I.arrow_forwardThe unknown compound with molecular formula C4H8O3, has infrared absorptions at 1710 and 2500 to 3100 cm^-1 and has ¹H NMR spectrum shown. Analyze the given data and propose a structure for this compound. Explain how you come up with your proposed compound.arrow_forwardCompound B has molecular formula C9H10. The IR spectrum is shown below. The 1H-NMR spectrum shows a multiplet at 7.2 ppm integrating to 4H, a triplet at 2.9 ppm integrating to 4H, and a triplet at 2.1 ppm integrating to 2 H. Suggest a structure for B and explain your reasoningarrow_forward
- The sex attractant of the housefly has the formula C23H46. When treated with warm potassium permanganate, this pheromone gives two products: CH3(CH2)12COOH and CH3(CH2)7COOH. Suggest a structure for this sex attractant. Explainwhich part of the structure is uncertainarrow_forwardCompound A exhibits the following H1 NMR, 13C NMR, and partial mass spectra respectively. When compound A is hydrolyzed, compound B is produced, Compound B exhibits the following H1 NMR, 13C NMR, and partial mass spectra respectively. Suggest structures for compounds A and B.arrow_forwardCompound A, C8H10O, has the IR and 1H NMR spectra shown. Propose a structure consistent with the observed spectra, and label each peak in the NMR spectrum. Note that the absorption at 5.5 î disappears when D2O is added.arrow_forward
- γ-Butyrolactone (C4H6O2, GBL) is a biologically inactive compound that is converted to the biologically active recreational drug GHB (Section 19.5) by a lactonase enzyme in the body. Since γ-butyrolactone is more fat soluble than GHB, it is more readily absorbed by tissues and thus produces a faster onset of physiological symptoms. γ-Butyrolactone shows an absorption in its IR spectrum at 1770 cm−1 and the following 1H NMR spectral data: 2.28 (multiplet, 2 H), 2.48 (triplet, 2 H), and 4.35 (triplet, 2 H) ppm. What is the structure of γ-butyrolactone?arrow_forwardA compound of formula C11H16N2 gives the IR, 1H NMR, and 13C NMR spectra shown. The proton NMR peak at δ 2 disappears on shaking with D2 Propose a structure for this compound, and show how your structure accounts for the observed absorptions.arrow_forwardOn being heated with a solution of sodium ethoxide in ethanol, compound A (C7H15Br) produced a mixture of two alkenes B and C, each of which had the molecular formula C7H14. Catalytic hydrogenation of major isomer B or minor isomer C gave only 3-ethylpentane. Suggest structures and mechanisms for compounds A, B, and C consistent with these observations.arrow_forward
- The treatment of (CH3)2C=CHCH2Br with H2O forms B (molecular formulaC5H10O) as one of the products. Determine the structure of B from its 1H NMR and IR spectra.arrow_forwardThymol (molecular formula C10H14O) is the major component of the oil ofthyme. Thymol shows IR absorptions at 3500–3200, 3150–2850, 1621, and1585 cm−1. The 1H NMR spectrum of thymol is given below. Propose apossible structure for thymol.arrow_forwardA compound C4H11N is known from its reactivity andspectroscopic properties to have no hydrogen atomsattached directly to the nitrogen atom. Write all structuralformulas consistent with this informationarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

