
Concept explainers
(a)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
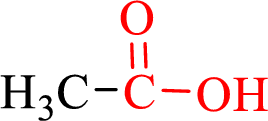
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the
alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(a)
Explanation of Solution
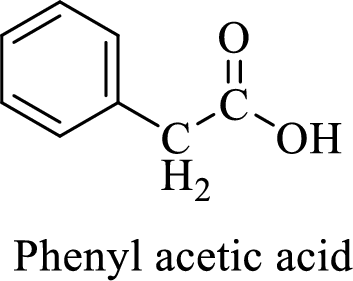
Name of the given compound is phenyl acetic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a carboxylic acid.
- ✓ A phenyl substituent is attached to the acetic acid; Acetic acid is the common name of ethanoic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
(b)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
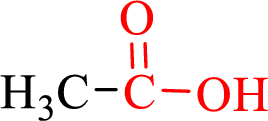
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(b)
Explanation of Solution
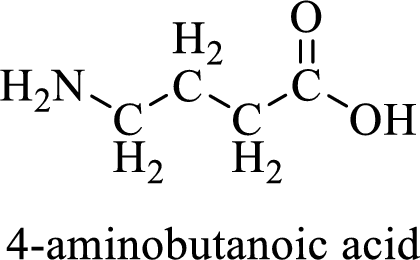
Name of the given compound is 4-Aminobutanoic acid
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a carboxylic acid.
- ✓ Parent chain contains four carbon atoms including the carboxylic acid functional group.
- ✓ An amino substituent is attached to the fourth carbon atom in the parent chain.
Thus,
The structural formula for this compound can be drawn as shown below,
(c)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
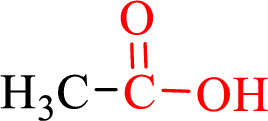
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(c)
Explanation of Solution
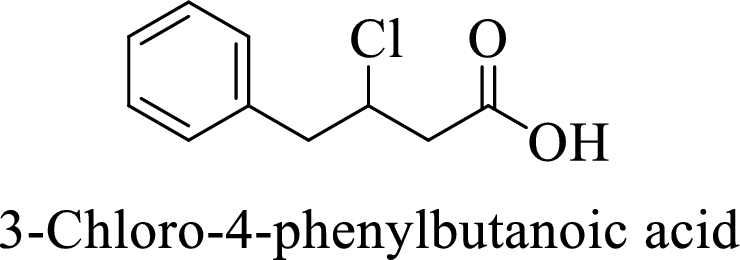
Name of the given compound is 3-Chloro-4-phenylbutanoic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a carboxylic acid.
- ✓ Parent chain contains four carbon atoms including the carboxylic acid functional group.
- ✓ One chlorine atom and a phenyl ring substituents are attached to the third and fourth carbon atoms in the parent chain respectively.
Thus,
The structural formula for this compound can be drawn as shown below,
(d)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
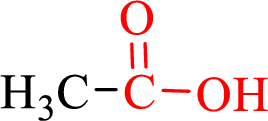
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(d)
Explanation of Solution
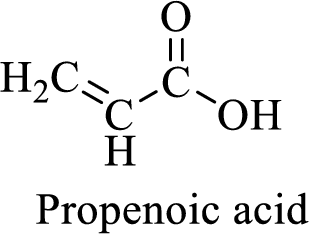
Name of the given compound is Propenoic acid (acrylic acid).
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a carboxylic acid.
- ✓ Parent chain contains three carbon atoms including the carboxylic acid functional group.
- ✓ There is a double bond in the compound.
Thus,
The structural formula for this compound can be drawn as shown below,
(e)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
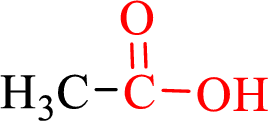
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
E-Z designators are used as like cis-trans terminology for non-similar groups attached
In E-Z designations, the groups attached to vinylic positions are checked by their priority on the basis of higher molecular weight. If the higher priority groups are on the same sides, then the configuration is designated as Z. If the higher priority groups are on the opposite sides, then the configuration is designated as E.
(e)
Explanation of Solution
Name of the given compound is (Z)-3-Hexenedioic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a dicarboxylic acid.
- ✓ Parent chain contains six carbon atoms including the carboxylic acid functional group.
- ✓ There is a double bond in the compound. The higher priority substituents are on same side; (Z)
Thus,
The structural formula for this compound can be drawn as shown below,
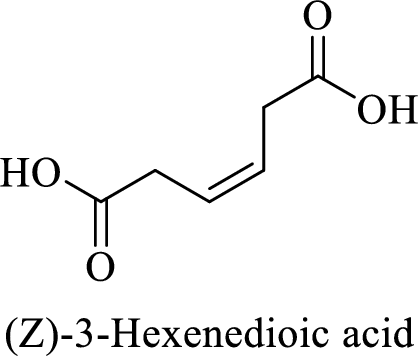
(f)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
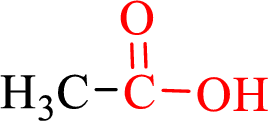
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(f)
Explanation of Solution
Name of the given compound is 2-Pentynoic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a monocarboxylic acid.
- ✓ Parent chain contains five carbon atoms including the carboxylic acid functional group.
- ✓ There is a triple bond in between second and third carbon atoms in the compound.
Thus,
The structural formula for this compound can be drawn as shown below,
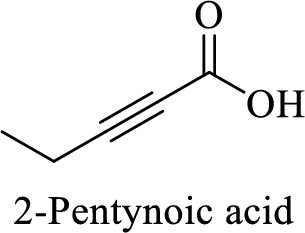
(g)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
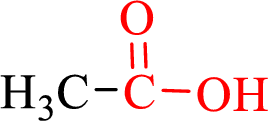
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(g)
Explanation of Solution
Name of the given compound is Potassium phenylacetate.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a potassium salt of phenyl acetic acid.
- ✓ A phenyl substituent is attached to the acetic acid; Acetic acid is the common name of ethanoic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
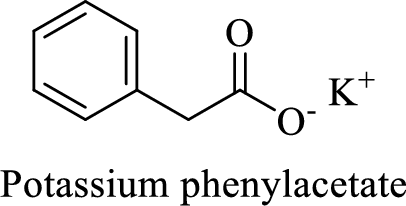
(h)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
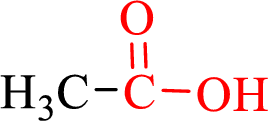
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(h)
Explanation of Solution
Name of the given compound is sodium oxalate.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a sodium salt of oxalic acid.
- ✓ Two sodium atoms are attached to the both carboxyl group of oxalic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
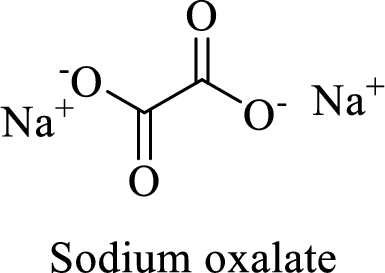
(i)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
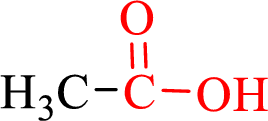
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(i)
Explanation of Solution
Name of the given compound is 2-Oxocyclohexanecarboxylic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a monocarboxylic acid.
- ✓ Parent ring contains six carbon atoms including the carboxylic acid functional group.
- ✓ There is a
ketone group on second carbon atom in the parent ring.
Thus,
The structural formula for this compound can be drawn as shown below,
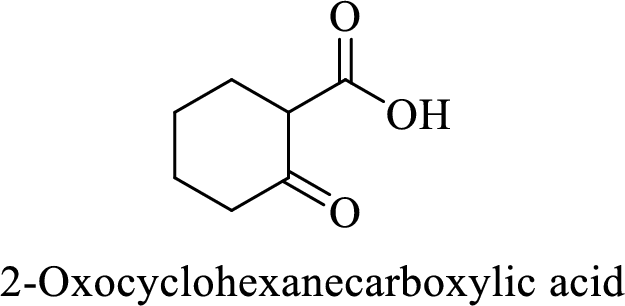
(j)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
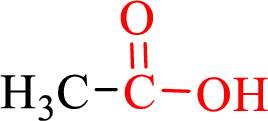
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
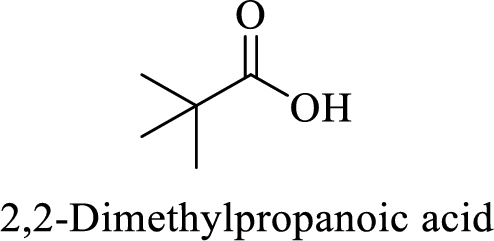
(j)
Explanation of Solution
Name of the given compound is 2, 2-Dimethylpropanoic acid.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a monocarboxylic acid.
- ✓ Parent chain contains three carbon atoms including the carboxylic acid functional group.
- ✓ Two methyl substituents are attached on the second carbon atom in the parent chain.
Thus,
The structural formula for this compound can be drawn as shown below,
Want to see more full solutions like this?
Chapter 17 Solutions
ORG.CHEM:TXT+OWLV2+MINDTAP 6MTHS >BI<
- When acetophenone, hydroxelamine hydrochloride, and sodium hydroxide are reacted to synthesize acetophenone oxime, why is it necessary to add hydrochloric acid after the reaction to make it acidic?arrow_forwardThe compound acetophenone has a very similar molar mass to that of benzoic acid and benzamide. However, acetophenone has a much lower m.p. (20 °C) than both such that, by contrast, it is a liquid at room temperature. By considering intermolecular forces and comparing functional group structure, account for this big difference in physical properties.arrow_forward1. Arrange the test samples in order of increasing reactivity with sodium bicarbonate? Explain briefly -Ethanoic acid, Butanoic acid, Benzoic acid 2. Arrange the test samples in order of increasing solubility in water? Explain briefly. -Ethylamine, Diethylamine, Aniline 3. Arrange the test samples in order of increasing basicity? Explain briefly. -NaOH, Ethylamine, Anilinearrow_forward
- Give the expected organic product when phenylacetic acid, PhCH2COOH, is treated with reagent Q.)NaHCO3, H2Oarrow_forwardWhen methanitrobenzoic acid is reacted with bromomethane in the presence of Lewis acids, which product occur.arrow_forwardGive the expected organic product when phenylacetic acid, PhCH2COOH, is treated with reagent Q.)LiAlH4 followed by H2Oarrow_forward
- In detail, explain how to separate a mixture of naphthalene, byphenyl-4-carboxylic acid, and 4-chloroaniline.arrow_forwardThe pKa, of the conjugate acid of morpholine is 8.33. (a) Calculate the ratio of morpholine to morpholinium ion in aqueous solution at pH 7.0. (b) At what pH are the concentrations of morpholine and morpholinium ion equal?arrow_forwardAs far back as the 16th century, South American Incas chewed the leaves of the coca bush, Erythroxylon coca, to combat fatigue. Chemical studies of Erythroxylon coca by Friedrich Wöhler in 1862 resulted in the discovery of cocaine, C17H21NO4, as the active component. Basic hydrolysis of cocaine leads to methanol, benzoic acid, and another compound called ecgonine, C9H15NO3. Oxidation of ecgonine with CrO3 yields a keto acid that readily loses CO2 on heating, giving tropinone. (a) What is a likely structure for the keto acid? (b) What is a likely structure for ecgonine, neglecting stereochemistry? (c) What is a likely structure for cocaine, neglecting stereochemistry?arrow_forward
- Identify X in the following equation, and explain how hexanoic acid (Section 19.2B) is formed by this stepwise reaction sequence.arrow_forwardThe pKa of acetamide (CH3CONH2) is 16. Draw the structure for its conjugate base and explain why acetamide is less acidic than CH3COOH.arrow_forwardCarnosine, found in muscle and brain tissue, acts as a buffer to neutralize small amounts of acid. The pKa of the conjugate acid of carnosine is close to 7.0. What is its structure?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


