
(a)
Interpretation:
The detailed mechanism for the given reaction is to be drawn and the major product of the reaction is to be predicted.
Concept introduction:
The addition reaction mechanism dominates on whether the nucleophile adds to the carbonyl carbon reversibly or irreversibly. Nucleophilic addition is irreversible or reversible depends on charge stability. Nucleophilic addition tends to be irreversible if the negative charge that develops in the adduct is substantially better stabilized than it is in the nucleophile. Nucleophilic addition to a carbonyl carbon tends to be irreversible when it involves a very strong nucleophile like
Answer to Problem 17.24P
The major product and mechanism for the given reaction are:
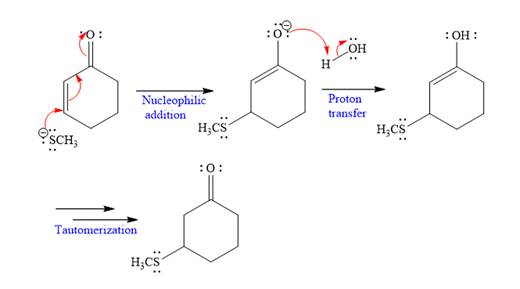
Explanation of Solution
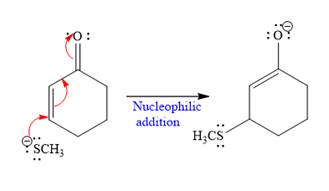
The nucleophile
In the first step,
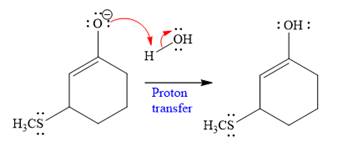
In the second step, this enolate anion is then protonated by an acid workup.
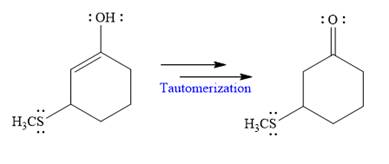
In the last step, tautomerization takes place and produces a ketone with a conjugate addition product.
Nucleophilic addition of
(b)
Interpretation:
The detailed mechanism for the given reaction is to be drawn and the major product of the reaction is to be predicted.
Concept introduction:
The addition reaction mechanism dominates on whether the nucleophile adds to the carbonyl carbon reversibly or irreversibly. Nucleophilic addition is irreversible or reversible depends on charge stability. Nucleophilic addition tends to be irreversible if the negative charge that develops in the adduct is substantially better stabilized than it is in the nucleophile. Nucleophilic addition to a carbonyl carbon tends to be irreversible when it involves a very strong nucleophile like
Answer to Problem 17.24P
The major product and detailed mechanism for the given reaction are:
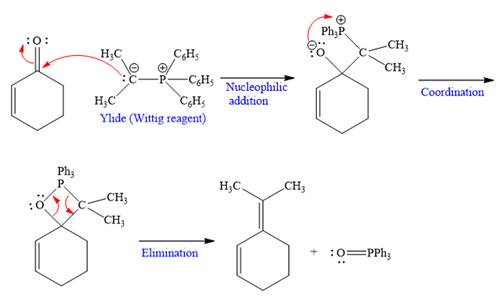
Explanation of Solution
The given nucleophile ylide is highly reactive and adds irreversibly to the
In the first step, the given highly reactive ylide attack on the carbonyl carbon of the
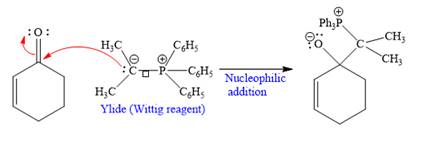
In the second step, the anion coordinates with
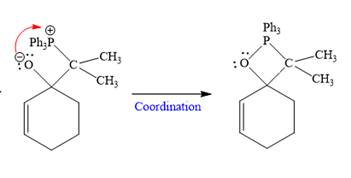
In the last step, Eliminates
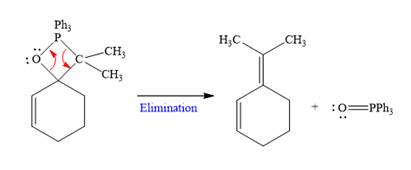
Nucleophilic addition of ylide (Wittig reagent) to the
(c)
Interpretation:
The detailed mechanism for the given reaction is to be drawn and the major product of the reaction is to be predicted.
Concept introduction:
The addition reaction mechanism dominates on whether the nucleophile adds to the carbonyl carbon reversibly or irreversibly. Nucleophilic addition is irreversible or reversible depends on charge stability. Nucleophilic addition tends to be irreversible if the negative charge that develops in the adduct is substantially better stabilized than it is in the nucleophile. Nucleophilic addition to a carbonyl carbon tends to be irreversible when it involves a very strong nucleophile like
Answer to Problem 17.24P
The major product and detailed mechanism for the given reaction are:
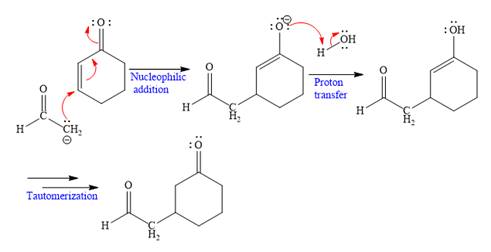
Explanation of Solution
The nucleophile
In the first step,
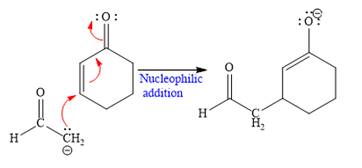
In the second step, this enolate anion is then protonated by an acid workup.
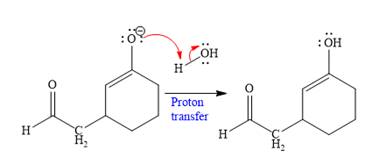
In the last step, tautomerization takes place and produces a ketone with a conjugate addition product.
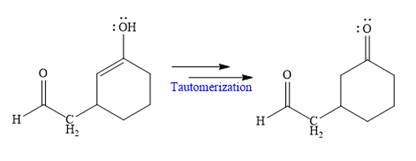
Nucleophilic addition of
(d)
Interpretation:
The detailed mechanism for the given reaction is to be drawn and the major product of the reaction is to be predicted.
Concept introduction:
The addition reaction mechanism dominates on whether the nucleophile adds to the carbonyl carbon reversibly or irreversibly. Nucleophilic addition is irreversible or reversible depends on charge stability. Nucleophilic addition tends to be irreversible if the negative charge that develops in the adduct is substantially better stabilized than it is in the nucleophile. Nucleophilic addition to a carbonyl carbon tends to be irreversible when it involves a very strong nucleophile like
Answer to Problem 17.24P
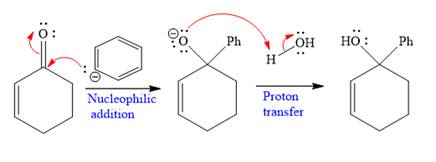
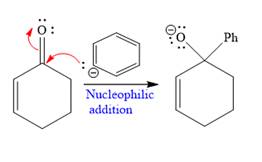
Explanation of Solution
The given nucleophile
In the first step,
In the second step, this anion is then protonated by acid workup and formed a direct addition product.
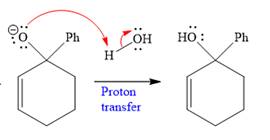
Nucleophilic addition of Grignard reagent to the
(e)
Interpretation:
The detailed mechanism for the given reaction is to be drawn and the major product of the reaction is to be predicted.
Concept introduction:
The addition reaction mechanism dominates on whether the nucleophile adds to the carbonyl carbon reversibly or irreversibly. Nucleophilic addition is irreversible or reversible depends on charge stability. Nucleophilic addition tends to be irreversible if the negative charge that develops in the adduct is substantially better stabilized than it is in the nucleophile. Nucleophilic addition to a carbonyl carbon tends to be irreversible when it involves a very strong nucleophile like
Answer to Problem 17.24P
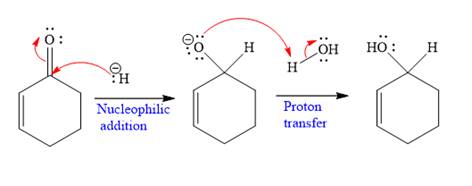
Explanation of Solution
The given nucleophile
In the first step,
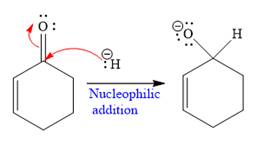
In the second step, this anion is then protonated by acid workup and formed a direct addition product.
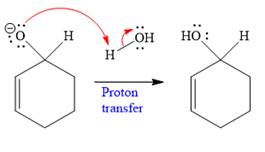
Nucleophilic addition of
Want to see more full solutions like this?
Chapter 17 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





