
Concept explainers
(a)
Interpretation:
The electrodes have to be labelled and the ions present in the solution has to be identified.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate
(a)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode.
The ions present in the solution are Lead, nitrate and Zinc.
The electrolytic cell with labelled electrodes and ions are shown below,
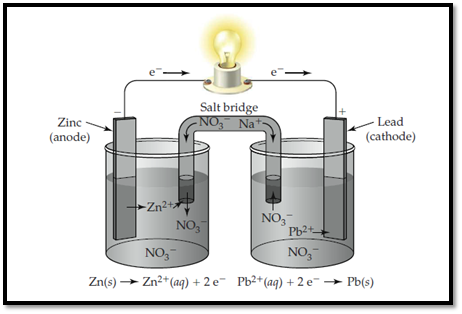
Figure 1
(b)
Interpretation:
The cathode and anode have to be labelled.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate electric current through the spontaneous redox reaction. The voltaic cell is an electrochemical cell, which is converting a chemical energy into electrical energy. Cathode as the positive electrode. Anode as the negative electrode. Salt bridge is required. Ions are discharged only in cathode while anode is consumed.
(b)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode. In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction.
The electrolytic cell with labelled electrodes is shown below,
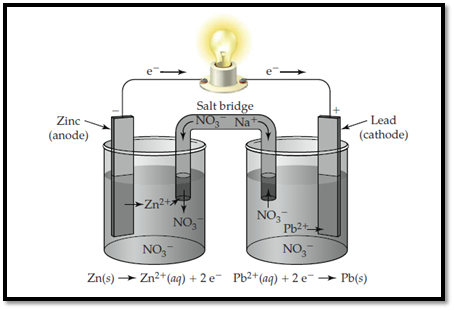
Figure 1
(c)
Interpretation:
The direction of flow of electron in the wire and ion flow in solution has to be indicated.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate electric current through the spontaneous redox reaction. The voltaic cell is an electrochemical cell, which is converting a chemical energy into electrical energy. Cathode as the positive electrode. Anode as the negative electrode. Salt bridge is required. Ions are discharged only in cathode while anode is consumed.
(c)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode. In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction. The flow of ions and electrons in the solution is shown below,
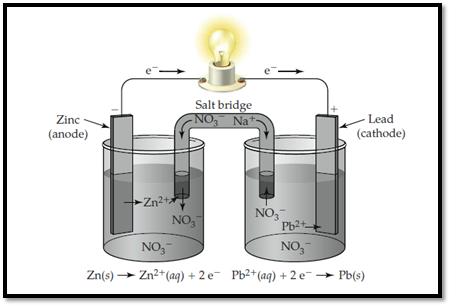
Figure 1
(d)
Interpretation:
The direction ion flow in solution and the electrolyte that is used for passage of ions has to be indicated.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate electric current through the spontaneous redox reaction. The voltaic cell is an electrochemical cell, which is converting a chemical energy into electrical energy. Cathode as the positive electrode. Anode as the negative electrode. Salt bridge is required. Ions are discharged only in cathode while anode is consumed.
(d)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode. In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction. The flow of ions and electrons in the solution is shown below. The passage of ions takes place with Sodium nitrate that acts as salt bridge.
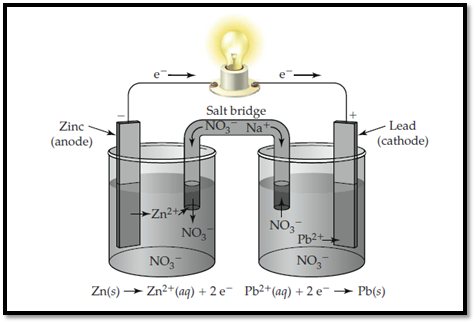
Figure 1
(e)
Interpretation:
The balance equation for the electrode and the overall cell reaction has to be given.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate electric current through the spontaneous redox reaction. The voltaic cell is an electrochemical cell, which is converting a chemical energy into electrical energy. Cathode as the positive electrode. Anode as the negative electrode. Salt bridge is required. Ions are discharged only in cathode while anode is consumed.
(e)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode. In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction. The flow of ions and electrons in the solution is shown below. The passage of ions takes place with Sodium nitrate that acts as salt bridge.
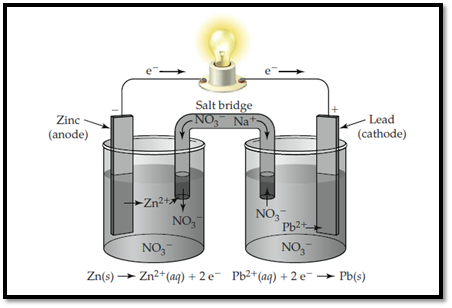
Figure 1
In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction.
The reactions at the cathode and anode is given as,
The overall reaction is given by summing up the cathode and anode reactions.
The overall reaction is written as,
Want to see more full solutions like this?
Chapter 17 Solutions
General Chemistry: Atoms First-Solution Manual
- The following two half-reactions arc involved in a voltaic cell. At standard conditions, what species is produced at each electrode? Ag++eAgE=0.80VNi2++2eNiE=0.25Varrow_forwardA potassium chloride solution is electrolyzed by passing a current through the solution using inert electrodes. A gas evolves at each electrode, and there is a large increase in pH of the solution. Write the half-reactions that occur at the anode and at the cathode.arrow_forwardThe mass of three different metal electrodes, each from a different galvanic cell, were determined before and after the current generated by the oxidation-reduction reaction in each cell was allowed to flow for a few minutes. The first metal electrode, given the label A, was found to have increased in mass; the second metal electrode, given the label B, did not change in mass; and the third metal electrode, given the label C, was found to have lost mass. Make an educated guess as to which electrodes were active and which were inert electrodes, and which were anode(s) and which were the cathode(s).arrow_forward
- An electrode is prepared from liquid mercury in contact with a saturated solution of mercury(I) chloride, Hg2Cl, containing 1.00 M Cl . The cell potential of the voltaic cell constructed by connecting this electrode as the cathode to the standard hydrogen half-cell as the anode is 0.268 V. What is the solubility product of mercury(I) chloride?arrow_forwardA solution contains the ions H+, Ag+, Pb2+, and Ba2+, each at a concentration of 1.0 M. (a) Which of these ions would be reduced first at the cathode during an electrolysis? (b) After the first ion has been completely removed by electrolysis, which is the second ion to be reduced? (c) Which, if any, of these ions cannot be reduced by the electrolysis of the aqueous solution?arrow_forwardFor each reaction listed, determine its standard cell potential at 25 C and whether the reaction is spontaneous at standard conditions. (a) Mn(s)+Ni2+(aq)Mn2+(aq)+Ni(s) (b) 3Cu2+(aq)+2Al(s)2Al3+(aq)+3Cu(s) (c) Na(s)+LiNO3(aq)NaNO3(aq)+Li(s) (d) Ca(NO3)2(aq)+Ba(s)Ba(NO3)2(aq)+Ca(s)arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





