
Concept explainers
Propose a structure consistent with each set of data.
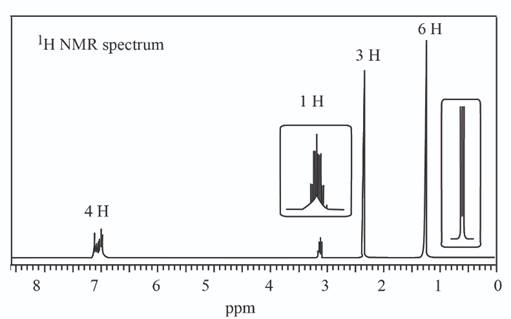
a.
b.
c.
(a)
Interpretation: A structure consistent with the given set of data is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 17.55P
A structure consistent with the given set of data is shown below.
Explanation of Solution
The given set of
Information from
The
Information from
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift value at
The possible structure of the compound based on the above analysis is,
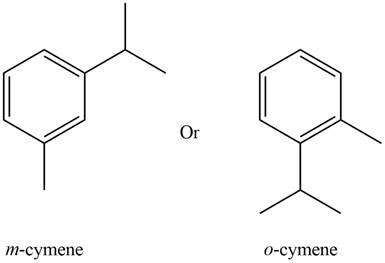
Figure 1
A structure consistent with the given set of data is shown in Figure 1.
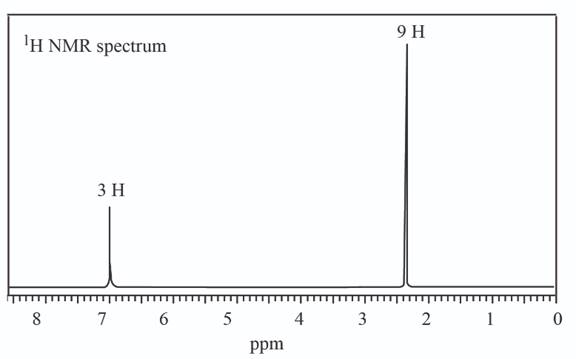
(b)
Interpretation: A structure consistent with the given set of data is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 17.55P
A structure consistent with the given set of data is shown below.
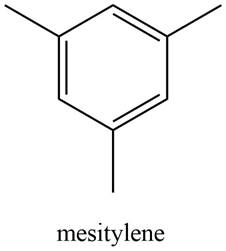
Explanation of Solution
The given set of
Information from
Three signals are observed in
The
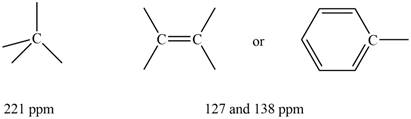
Figure 2
Information from
The observed chemical shift value at
The observed chemical shift value at
The possible structure of the compound based on the above analysis is,

Figure 3
A structure consistent with the given set of data is shown in Figure 3.
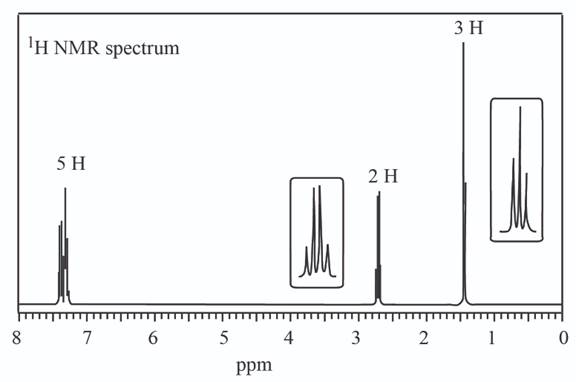
(c)
Interpretation: A structure consistent with the given set of data is to be predicted.
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 17.55P
A structure consistent with the given set of data is shown below.
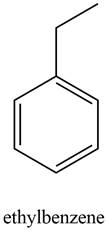
Explanation of Solution
The given set of
Information from IR data:
The
Information from
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift value at
The possible structure of the compound based on the above analysis is,
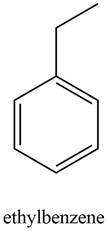
Figure 4
A structure consistent with the given set of data is shown in Figure 4.
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY
- The mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this hydrocarbon, and explain the spectral data.arrow_forwardThe 1H and 13C NMR spectra of compound A, C8H9Br, are shown. Propose a structure for A, and assign peaks in the spectra to your structure.arrow_forwardCompound A, C8H10O, has the IR and 1H NMR spectra shown. Propose a structure consistent with the observed spectra, and label each peak in the NMR spectrum. Note that the absorption at 5.5 î disappears when D2O is added.arrow_forward
- Propose possible structures consistent with each set of data. Assume each compound has an sp3 hybridized C – H absorption in its IR spectrum, and that other major IR absorptions above 1500 cm are listed. a. a compound having a molecular ion at 72 and an absorption in its IR spectrum at 1725 cm−1 b. a compound having a molecular ion at 55 and an absorption in its IR spectrum at ~2250 cm−1 c. a compound having a molecular ion of 74 and an absorption in its IR spectrum at 3600–3200 cm−1arrow_forwardPropose structures for the compound C5H10O whose 1H NMR spectra is belowarrow_forwardGiven the following NMR data, find the unknown compound. a. Identify the molecular formula; find IHDb. Identify the 1H and 13C NMR signalsarrow_forward
- The NMR spectra for compound 1 were acquired in a 7.5 mg / 0.6 mL solution ofCDCl3 . The 1H and 13C peaks are also listedbelow. Provide a full analysis of the NMR spectra for compound 1. correct assignment of NMR spectra of both 13C spectra. correct rationalisation of 13C spectrum1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 9.5 Hz, 1H), 7.56 (ddd, J = 8.5, 7.5, 1.6 Hz, 1H),7.51 (dd, J = 7.5, 1.6 Hz, 1H), 7.36 (d, J = 8.5 Hz, 1H), 7.30 (dd, J = 8.5, 7.5 Hz, 1H), 6.45(d, J = 9.5 Hz, 1H).13C NMR (101 MHz, CDCl3) δ 160.79, 154.09, 143.43, 131.85, 127.87, 124.44, 118.86,116.94, 116.74.Note: There are two carbon peaks in the 13C spectrum that are so close together that they are not differentiable at the resolution in this experiment. you should be able to assign these peaks to one of two carbon atoms in 1.arrow_forwardIdentify the carbon atoms that give rise to each NMR signal.arrow_forward35. Compounds A. and B. are closely related and have formulas of C₁0H₁2O and C₁1H140 respectively. Both show an IR absorption at 1710 cm-¹. Based on NMR of A. and B. provide structures for each a. - ¹H NMR t S 2 11 10 Integrals 9 T m, (overlapped resonances) 5 8 7 6 5 ppm 4 3 2 T -N 2 T 3 1 0arrow_forward
- Propose possible structures consistent with each set of data. Assume each compound has an sp3 hybridized C—H absorption in its IR spectrum, and that other major IR absorptions above 1500 cm−1 are listed. a.a compound having a molecular ion at 72 and an absorption in its IR spectrum at 1725 cm−1 b. a compound having a molecular ion at 55 and an absorption in its IR spectrum at −2250 cm−1 c.a compound having a molecular ion at 74 and an absorption in its IR spectrum at 3600−3200 cm−1arrow_forwardCompound C has molecular formula C5H8O. The IR, mass, 1H-NMR, and 13C-NMR spectra are shown below. Suggest a structure for C and explain your reasoning.arrow_forwardPlease help annotate all of the spectra in the picture below, thank you so so much! It is C NMR, H NMR, and MS!arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

