
Concept explainers
(a)
Interpretation:
The synthesis of the given
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is shown below:
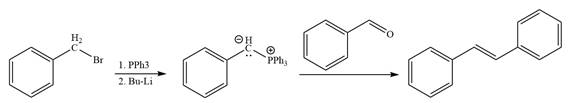
Explanation of Solution
The given structure of alkene is
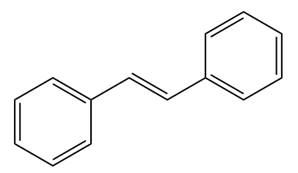
In the above structure, the
By undoing a Wittig reaction in retrosynthetic analysis,
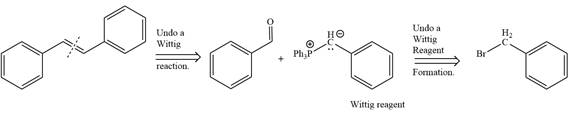
In the forward direction, the synthesis of the given alkene from an alkyl halide and benzaldehydewould appears as follows:
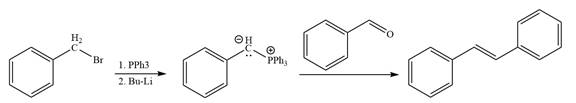
The synthesis of given alkene from an alkyl halide and a ketone or aldehyde is shown.
(b)
Interpretation:
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is to be shown.
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or aldehyde by two different ways as shown below.
Method 1:
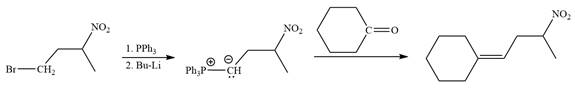
Method 2:
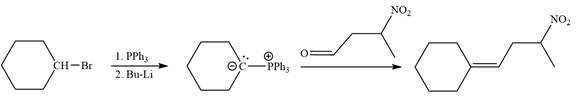
Explanation of Solution
The given structure of alkene is
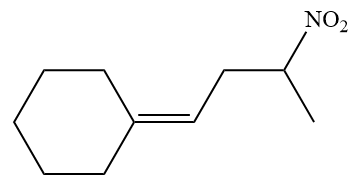
In the above structure, the
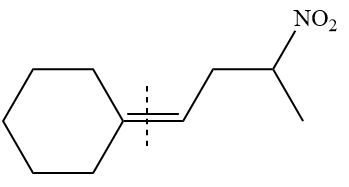
Method 1:
By undoing a Wittig reaction in retrosynthetic analysis,
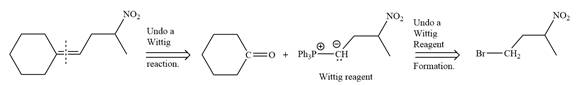
In the forward direction, the synthesis of the given alkene from an alkyl halide and a ketone would appear as follows:
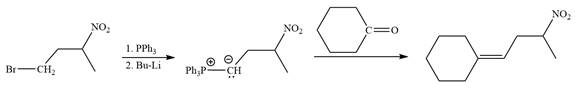
Method 2:
By undoing a Wittig reaction in retrosynthetic analysis,
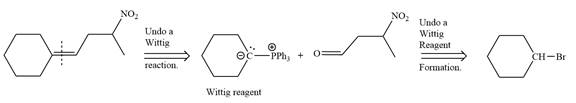
In the forward direction, the synthesis of the given alkene from an alkyl halide and an aldehyde would appear as follows:

The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or an aldehyde by two different ways is shown.
(c)
Interpretation:
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is to be shown.
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or aldehyde by two different ways as shown below.
Method 1:
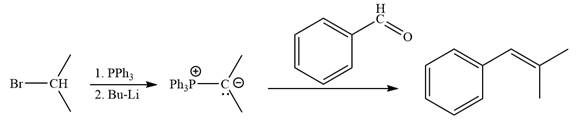
Method 2:
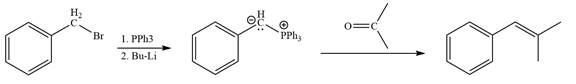
Explanation of Solution
The given structure of alkene is
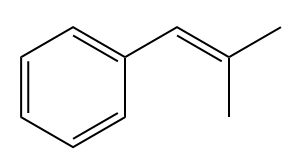
In the above structure, the
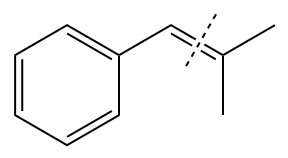
Method 1:
By undoing a Wittig reaction in retrosynthetic analysis,
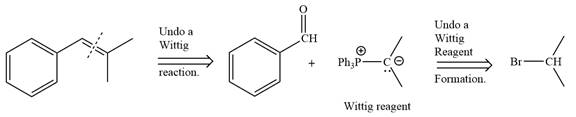
In the forward direction, the synthesis of the given alkene from an alkyl halide and benzaldehyde would appear as follows:

Method 2:
By undoing a Wittig reaction in retrosynthetic analysis,
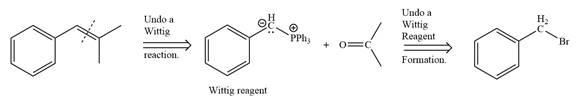
In the forward direction, the synthesis of the given alkene from an alkyl halide and a ketone would appear as follows:

The synthesis of the given unsymmetric alkene from an alkyl halide and a ketone or an aldehyde by two different ways is shown.
Want to see more full solutions like this?
Chapter 17 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- (SYN) Show how to carry out each of the following syntheses, using any reagents necessary. Hint: In each case, the carbonyl group of a ketone or aldehyde is entirely removed. (a) (b) ? OCH3 OCH3 OCH3 OCH3 (c) (d) ? ? OH Pharrow_forwardBy How do the following reactions, pleases Propose Mechanism?arrow_forwardA student attempted to synthesize an epoxide according to the reaction scheme shown here, but no epoxide was formed. Explain why. Hint: It may be helpful to build a OH NaOH No epoxide model of the reactant molecule. (H3C);C' Brarrow_forward
- In the acid-catalyzed aromatic alkylation involving 1-methylcyclohexene and benzene, two isomeric products are possible, but only one is formed, as shown here. Draw the complete mechanism that leads to each product, and explain why only one isomer is formed.arrow_forward(SYN) Provide the reagents necessary to perform the following transformation. OH A Вarrow_forwardPlease synthesize each of the compounds shown below from benzene.arrow_forward
- Please answer completely will give rating surelyarrow_forwardDraw the complete, detailed E1 mechanism for each of the following reactions, and show all resonance structures, where applicable.arrow_forward(SYN) Show how to synthesize each of the following trisubstituted benzenes from a disubstituted benzenearrow_forward
- Problem: Show how the following compound can be produced from an alkene. Include the alkene, the reagents, and any special reaction conditions. Pay attention to stereochemistry.arrow_forwardProvide the complete mechanism using curved arrow formalism for the reaction of pyruvic acid treated with the compound shown below>arrow_forwardHelp me pleasearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
