
Concept explainers
Practice Problem 17.1
Give an IUPAC systematic name for each of the following:
(a)
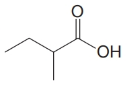
(b)
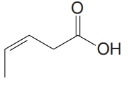
(c)
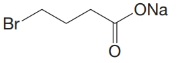
(d)
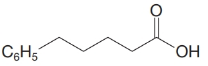
(e)
Interpretation:
The IUPAC name for each given carboxylic compound is to be identified.
Concept introduction:
The carboxylic group
Answer to Problem 1PP
Solution:
Explanation of Solution
a)

Start numbering the chain beginning from the carbon of the carboxylic group.
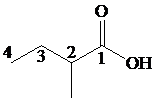
This chain contains four carbons and so the base name is butanoic acid. In the position-2, it has a methyl group attached. Therefore, the compound is
b)
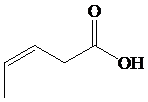
The numbering of the chain begins from the carbon of the carboxylic group.
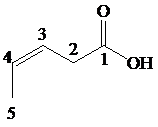
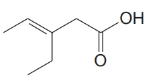
This chain contains five carbons. So, the compound belongs to pentanoic acid. Here, in position-3, it has a double bond. Thus, by considering this double bond and its stereochemistry, the compound identified is
c)

The numbering of the chain begins from the carbon of the carboxylic group.
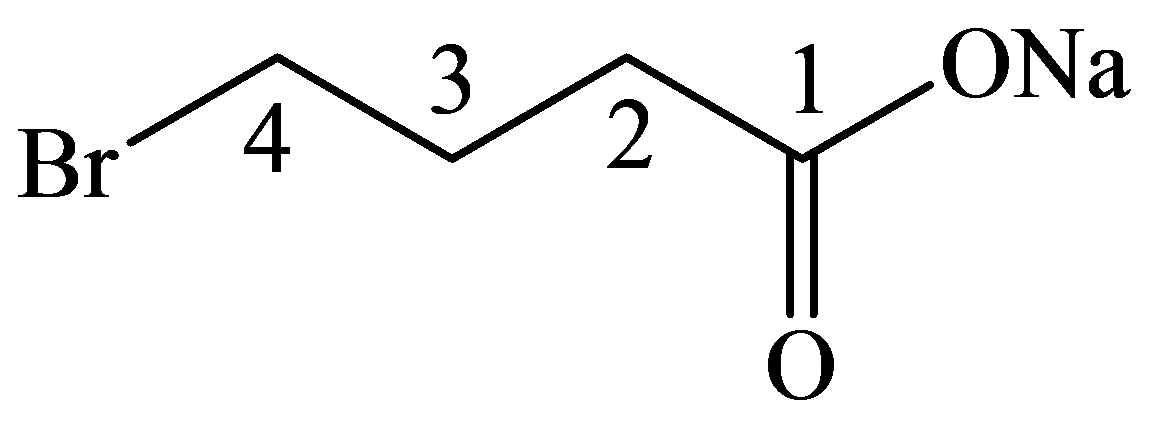
This chain contains four carbons, and so, the name of the compound must be butane. Here, the hydrogen of the carboxylic group is replaced by sodium ion
d)
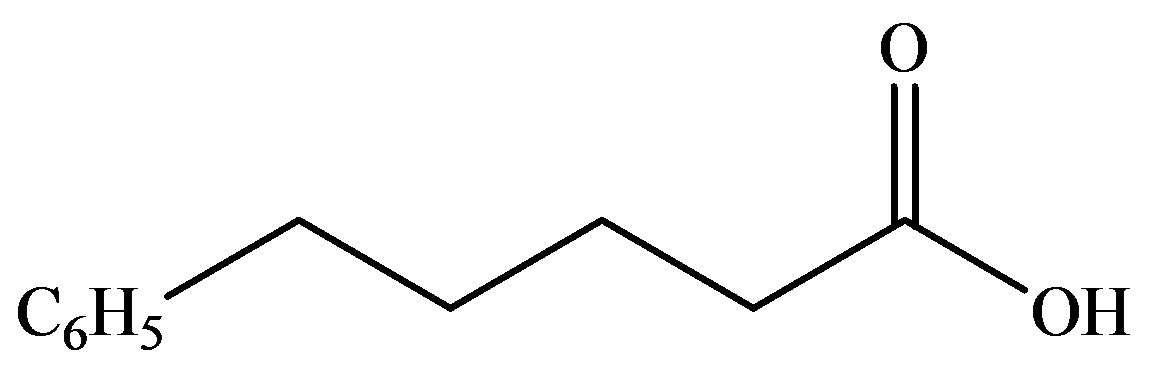
The numbering of the chain begins from the carbon of the carboxylic group.
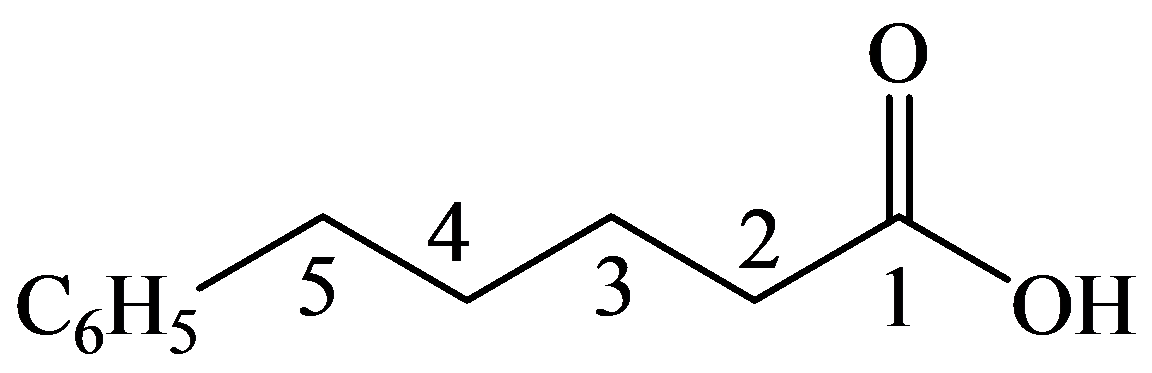
This chain contains five carbons, and so, it is pentanoic acid. It has a phenyl compound at position-5. Thus, the name of the compound is
e)

The numbering of the chain begins from the carbon of the carboxylic group.
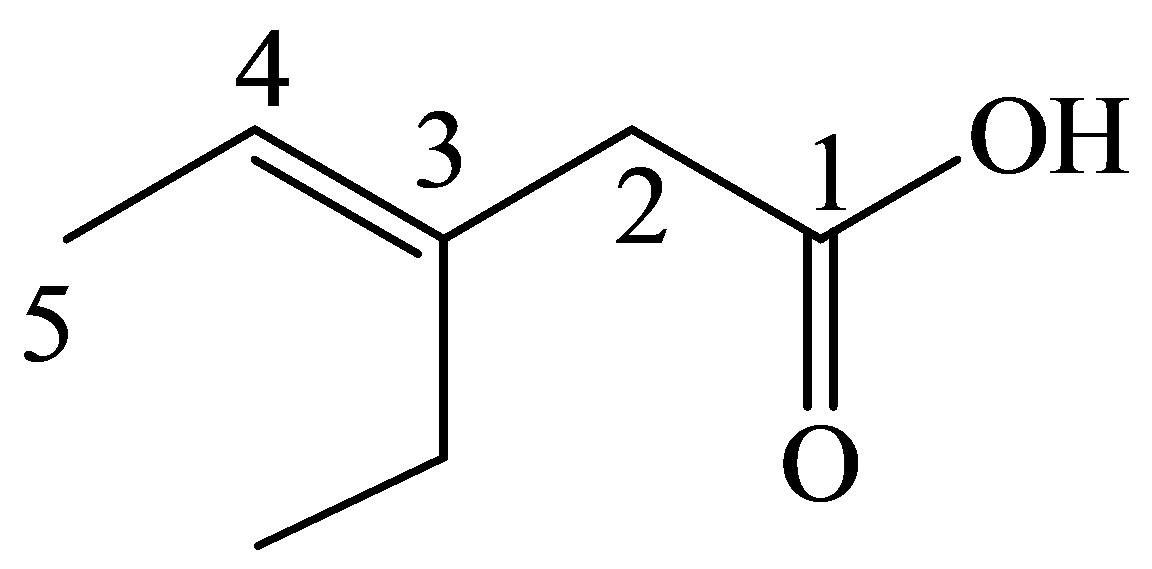
This chain contains five carbons. So, the name is pentanoic acid. There is a double bond and an ethyl group in position-3. Therefore, by considering the above conditions and the stereochemistry involved, the name of the compound is
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEM LL W/WILEYPLUS BLCKBRD >I
Additional Science Textbook Solutions
Chemistry: A Molecular Approach (4th Edition)
Chemistry
Chemistry & Chemical Reactivity
Chemistry (7th Edition)
Principles of Chemistry: A Molecular Approach (3rd Edition)
General Chemistry: Principles and Modern Applications (11th Edition)
- give the structural formula for compounds A to Garrow_forwardCompound A is an alcohol that undergoes oxidation to produce compound B.Compound B is a ketone that gives positive triiodomethane reaction. Compound B isthen reacted with phenyl magnesium bromide, C6H5MgBr in the presence of aqueousacid to form compound C. Compound C has the molecular formula of C9H12O. Deducethe structure for compound A, B and C. PLEASE PROVIDE CLEAR DRAWINGS AND EXPLANATIONSarrow_forwardCompound A, C3H7Br, does not react with cold dilute potassium permanganate solution. Upon treatment with potassium hydroxide in ethanol, A gives only product B, C3H6. Unlike A, B decolourises potassium permanganate solution. Ozonolysis of Bgives C, C2H4O, and D, CH2O. Suggest the structural formulae of A, B, C and D.Write the equations for all the reactions involved.arrow_forward
- Compound A is an aromatic compound with the molecular formula C8H8. When treated with excess BR2, compound A is converted into compound B, with the molecular formula C8H8BR2. Draw the structure of compound a. arrow_forwardOutline a synthesis for the following transformation and provide a justification for your chosen strategy. (More than one steps may be required)arrow_forwardWhen the nitrogen-containing aromatic heterocyclic compounds 1 and 2 are treated with HCl, only 1 forms the hydrochloride salt, whereas compound 2 is unreactive. Provide an explanation for this observed reactivity.arrow_forward
- Give the IUPAC name for the following compound. Be sure to indicate stereochemistry where necessary.arrow_forwardQ1. Outline two methods of phenols synthesis. Q2. Give a concise account on the nomenclature of heterocyclic compound.arrow_forwardGive mechanistic explanations for of the following synthetic transformations. You should explain any regio- or stereochemical control observed as appropriate.arrow_forward
- Two moles of organic compound ‘A’ on treatment with a strong base gives two compounds ‘B’ and ‘C’. Compound ‘B’ on dehydrogenation with Cu gives ‘A’ while acidification of ‘C’ yields carboxylic acid ‘D’ with molecular formula of CH2O2. Identify the compounds A, B, C and D and write all chemical reactions involved.arrow_forward-Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the molecular formula C10H16 and a UV absorption maximum at 232 nm. On hydrogenation with a palladium catalyst, 2,6-dimethyloctane is obtained. Ozonolysis of -ocimene, followed by treatment with zinc and acetic acid, produces the following four fragments: (a) How many double bonds does -ocimene have? (b) Is -ocimene conjugated or nonconjugated? (c) Propose a structure for -ocimene. (d) Write the reactions, showing starting material and products.arrow_forwardTriphenylmethanol is insoluble in water, but when it is treated with concentrated sulfuric acid, a bright yellow solutionresults. As this yellow solution is diluted with water, its color disappears and a precipitate of triphenylmethanol reappears.Suggest a structure for the bright yellow species, and explain this unusual behavior.1arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


