
Interpretation:
The principal organic products formed in each of the given reactions are to be identified.
Concept Introduction:
In the
The naming of chiral center and geometric isomers are based on Cahn-Ingold-Prelog priority rules. According to these rules, if the priority assigned to each group attached to the chirality center in a molecule is in a clockwise direction, then it is the R-stereoisomer.
Tosylate is a good leaving group. Tosylate leaves a pair of electrons followed by the nucleophilic attack on the reacting molecule to form a new
Lithium aluminum hydride is a strong reducing agent. Alcohols or ethers are the functional groups that involve
Oxirane that is commonly called as ethylene oxide is a cyclic ether. Oxirane that is commonly called as ethylene oxide is a cyclic ether.
Answer to Problem 26P
Solution:
a) The principal organic products of the given reaction are as follows:
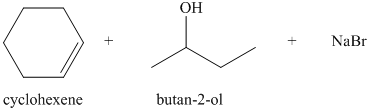
b) The principal organic products of the given reaction are as follows:
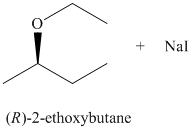
c) The principal organic product of the given reaction is as follows:
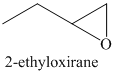
d) The principal organic product of the given reaction is as follows:
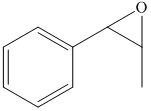
e) The principal organic product of the given reaction is as follows:
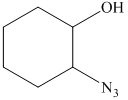
f) The principal organic product of the given reaction is as follows:
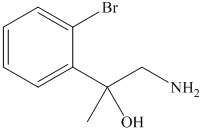
g) The principal organic product of the given reaction is as follows:
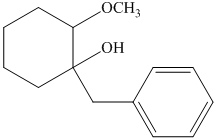
h) The principal organic product of the given reaction is as follows:
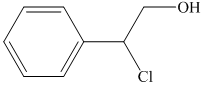
i) The principal organic product of the given reaction is as follows:
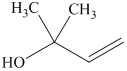
j) The principal organic product of the given reaction is
k) The principal organic product of the given reaction is as follows:
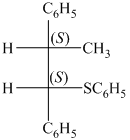
Explanation of Solution
a) Principle organic product formed in the given reaction.
The starting reagents of the given reaction are bromocyclohexane and sodium butan-2-olate. Sodium butan-2-olate is a strong base and bromocyclohexane is a secondary alkyl bromide. Therefore,
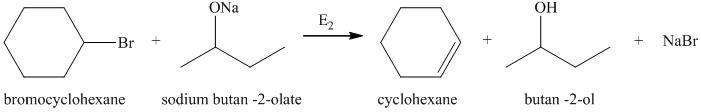
b) Principle organic product formed in the given reaction.
The starting reagents of the given reaction are iodoethane and sodium-(R)-butan-2-olate. They react with each other to form (R)-2-ethoxybutane and sodium iodide. The complete chemical reaction is shown below.
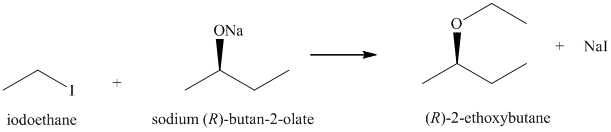
c) Principle organic product formed in the given reaction.
The starting reagent of the given reaction is a halohydrin, 1-bromobutan-2-ol which reacts with a base to form 2-ethyloxirane. The complete chemical reaction is shown below.
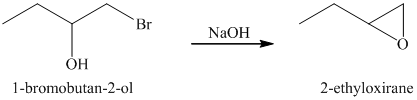
d) Principle organic product formed in the given reaction.
The starting reagent of the given reactions are prop-1-enylbenzene and perbenzoic acid. They react with each other to form 2-methyl-3-phenyloxirane. The complete chemical reaction is shown below.
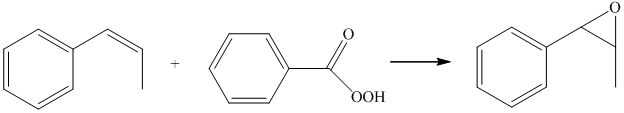
e) Principle organic product formed in the given reaction.
The starting reagent of the given reactions is an
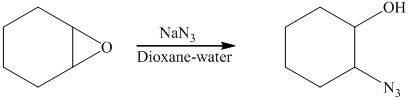
f) Principle organic product formed in the given reaction.
In the presence of the base ammonia, opening of the epoxide ring takes place at less substituted carbon. The complete chemical reaction is shown below.
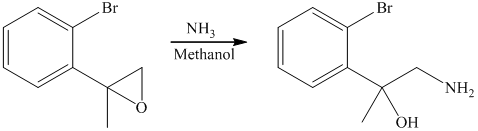
g) Principle organic product formed in the given reaction.
Sodium methoxide is a strong base and in the presence of this strong base and methanol, opening of the epoxide ring takes place at less substituted carbon. The complete chemical reaction is shown below.
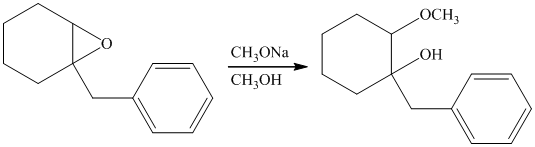
h) Principle organic product formed in the given reaction.
Hydrochloric acid is a strong acid and in the presence of this strong acid and
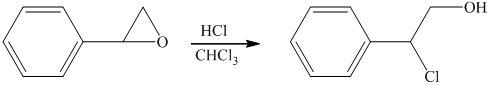
i) Principle organic product formed in the given reaction.
Lithium aluminium hydride is a strong base and in the presence of this strong base and diethyl ether, opening of the epoxide ring takes place at less substituted carbon. The complete chemical reaction is shown below.
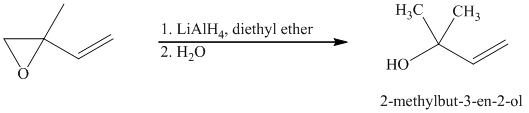
j) Principle organic product formed in the given reaction.
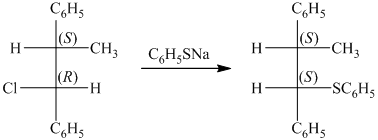
The given starting material is a tosylate. It is a good leaving group. It leaves with a pair of electrons followed by the nucleophilic attack on sodium butane thiolate to form a new
k) Principle organic product formed in the given reaction.
The given starting material is a secondary alkyl halide. It reacts with
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Predict the major organic product for the following reaction sequence, and then determine the stereochemical nature of the final product.arrow_forwardPredict the principal organic products for each of the following reactions:arrow_forwardPredict the major organic product of each of the following reactions or provide the reagent needed to complete each transformation.arrow_forward
- Predict the major organic product of the following reactions, including the stereochemistry where it is applicable.arrow_forwardGive the major organic product(s) for each of the following reactionsarrow_forwardPredict the major product of the following reactions. If it is possible, write all stereoisomers.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





