
(a)
The work done on the gas in an isothermal process.
(a)
Answer to Problem 52P
Thework done on the gas in an isothermal process is
Explanation of Solution
Write the expression for the work done on the gas,
Here,
For the isothermal process, the work done on the gas,
Here,
Conclusion:
Substitute
Therefore, the work done on the gas in an isothermal process is
(b)
Thework done on the gas in an adiabatic process.
(b)
Answer to Problem 52P
Thework done on the gas in an adiabatic process is
Explanation of Solution
Write the expression for the temperature of the system at point
Here,
Write the expression for the molar specific heat at constant volume,
Here,
Write the expression for the work done on the gas in an adiabatic process,
Here,
Conclusion:
Substitute
Substitute
Substitute
Therefore, the work done on the gas in an adiabatic process is
(c)
The final pressure of the gas in an isothermal process.
(c)
Answer to Problem 52P
The final pressure of the gas in an isothermal process is
Explanation of Solution
Write the expression for the
For the isothermal process,
Here,
Conclusion:
Substitute
Therefore, the final pressure of the gas in an isothermal process is
(d)
The final pressure of the gas in an adiabatic process.
(d)
Answer to Problem 52P
The final pressure of the gas in an adiabatic process is
Explanation of Solution
For the adiabatic process,
Write the expression for the final pressure of the gas,
Conclusion:
Substitute
Therefore, the temperature at the end of the cycle is
Want to see more full solutions like this?
Chapter 17 Solutions
Principles of Physics: A Calculus-Based Text, Hybrid (with Enhanced WebAssign Printed Access Card)
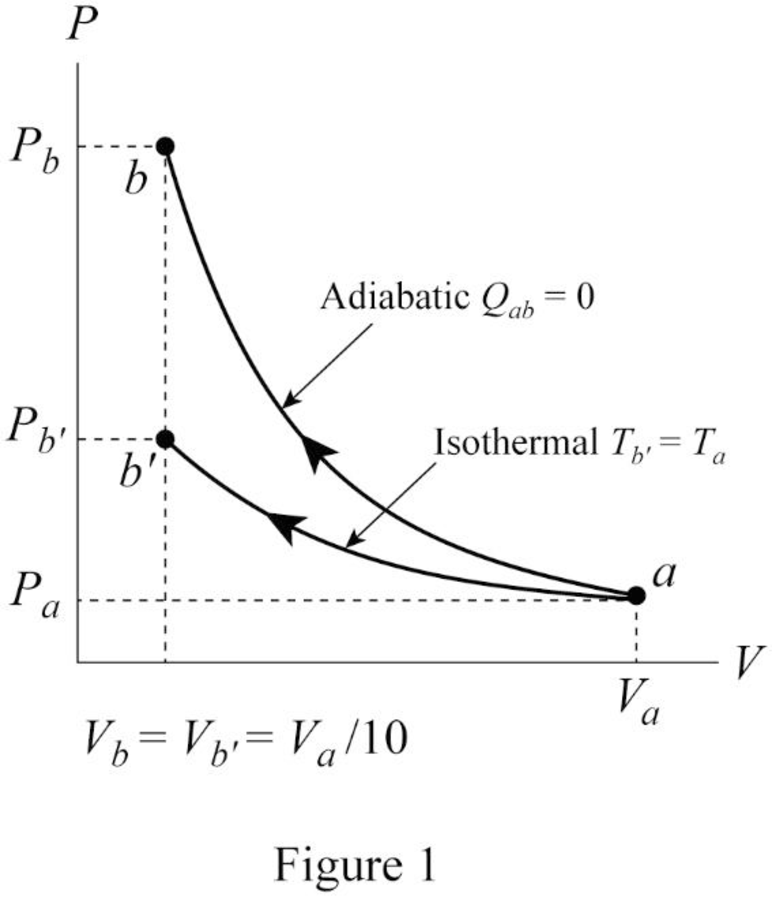
- The energy input to an engine is 3.00 times greater than the work it performs. (i) What is its thermal efficiency? (a) 3.00 (b) 1.00 (c) 0.333 (d) impossible to determine (ii) What fraction of the energy input is expelled to the cold reservoir? (a) 0.333 (b) 0.667 (c) 1.00 (d) impossible to determinearrow_forwardHow much work is required to compress 5.00 mol of air at 20.0C and 1.00 atm to one-tenth of the original volume (a) by an isothermal process? (b) What If? How much work is required to produce the same compression in an adiabatic process? (c) What is the final pressure in part (a)? (d) What is the final pressure in part (b)?arrow_forwardA car tile contains 0.0380 m3 of air at a pressure of 2.20105 Pa (about 32 psi). How much more internal energy does this gas have than the same volume has at zero gauge pressure (which is equivalent to normal atmospheric pressure)?arrow_forward
- An ideal gas initially at 300 K undergoes an isobaric expansion at 2.50 kPa. If the volume increases from 1.00 m3 to 3.00 m3 and 12.5 kJ is transferred to the gas by heat, what are (a) the change in its internal energy and (b) its final temperature?arrow_forwardDuring the power stroke in a four-stroke automobile engine, the piston is forced down as the mixture of combustion products and air undergoes an adiabatic expansion. Assume (1) the engine is running at 2 500 cycles/min; (2) the gauge pressure immediately before the expansion is 20.0 atm; (3) the volumes of the mixture immediately before and after the expansion are 50.0 cm3 and 400 cm3, respectively (Fig. P21.31); (4) the time interval for the expansion is one-fourth that of the total cycle; and (5) the mixture behaves like an ideal gas with specific heat ratio 1.40. Find the average power generated during the power stroke.arrow_forwardTwo moles of a monatomic ideal gas such as oxygen is compressed adiabatically and reversibly from a state (3 atm, 5 L) to a state with a pressure of 4 atm. (a) Find the volume and temperature of the final state. (b) Find the temperature of the initial state. (c) Find work done by the gas in the process. (d) Find the change in internal energy in the process. Assume Cv=5R and Cp=Cv+R for the diatomic ideal gas in the conditions given.arrow_forward
- A monatomic ideal gas undergoes a quasi-static adiabatic expansion in which its volume is doubled. How is the pressure of the gas changed?arrow_forwardOne mole of an ideal gas does 3 000 J of work on its surroundings as it expands isothermally to a final pressure of 1.00 atm and volume of 25.0 L. Determine (a) the initial volume and (b) the temperature of the gas.arrow_forwardDoes the temperature of an ideal gas increase, decrease, or stay the same during (a) an isothermal expansion, (b) an expansion at constant pressure, (c) an adiabatic expansion, and (d) an increase in pressure at constant volume?arrow_forward
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning



