
Concept explainers
(a)
Interpretation: Efficient synthesis for the given transformation has to be proposed.
Concept Introduction:
Bromination: In bromination reaction, hydrogen atom of a molecule is replaced by a bromine atom.
In a reaction,
(b)
Interpretation: Efficient synthesis for the given transformation has to be proposed.
Concept Introduction:
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
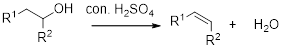
Alcohol is reaction with concentrated sulfuric acid, first alcohol gets protonated forms carbocation (more stable carbocation) followed by elimination of proton (
Tertiary carbocation is more stable than the secondary, secondary carbocation is more stable than primary.

In dehydration reaction, sulfuric acid is act as a proton donor, and which is used to protonate the alcohol and makes carbocation therefore sulfuric acid is the driving force of the reaction. Dehydration reaction will not go without acid (sulfuric acid).
Hydroboration reaction: The reaction involves addition of
Anti-Markovnikov’s Addition Rule: The unsymmetrical alkene in a chemical compound reacts with hydrogen halide in a way, where halide ions attacks and bond to the less substitution position of carbon-carbon double bond.
In a reaction, fuming sulfuric acid is used to introduce sulfonic groups in a compound (sulfonation) or as dehydrating agent (removing of water molecule).
(c)
Interpretation: Efficient synthesis for the given transformation has to be proposed.
Concept Introduction:
Structure of the substrate plays major role in the reactivity of
In a reaction,
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
ORGANIC CHEMISTRYPKGDRL+MLCRL MDL
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





