
(a)
Interpretation:
The structure of amide formed by the reaction of propylamine
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In
Answer to Problem 60P
The propanoic acid
Explanation of Solution
The reaction of carboxylic acid with ammonia or amines forms amide molecules. It involves the formation of water molecule. In this reaction the amine nitrogen atom react with carbonyl carbon atom of carboxylic acid to form amide.
In the reaction of
(b)
Interpretation:
The structure of amide formed by the reaction of
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Amines are the organic compounds with general chemical formula of R-NH2 or R-NH-R whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Answer to Problem 60P
The Dodecanoic acid
Explanation of Solution
The reaction of carboxylic acid with ammonia or amines forms amide molecules. It involves the formation of water molecule. In this reaction the amine nitrogen atom react with carbonyl carbon atom of carboxylic acid to form amide.
In the reaction of
(c)
Interpretation:
The structure of amide formed by the reaction of
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Amines are the organic compounds with general chemical formula of R-NH2 or R-NH-R whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Answer to Problem 60P
The formic acid
Explanation of Solution
The reaction of carboxylic acid with ammonia or amines forms amide molecules. It involves the formation of water molecule. In this reaction the amine nitrogen atom react with carbonyl carbon atom of carboxylic acid to form amide.
In the reaction of
(d)
Interpretation:
The structure of amide formed by the reaction of p-methoxybenzoic acid with
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Amines are the organic compounds with general chemical formula of R-NH2 or R-NH-R whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Answer to Problem 60P
The p-methoxybenzoic acid reacts with
Explanation of Solution
The reaction of carboxylic acid with ammonia or amines forms amide molecules. It involves the formation of water molecule. In this reaction the amine nitrogen atom react with carbonyl carbon atom of carboxylic acid to form amide.
In the reaction of
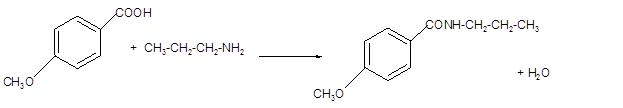
Want to see more full solutions like this?
Chapter 17 Solutions
GEN ORG + BIO (LL) W/CONNECT
- Draw the structure of a compound of molecular formula C4H11NO that fits each description: (a) a compound that contains a 1° amine and a 3° alcohol; (b) a compound that contains a 3° amine and a 1° alcohol.arrow_forwardWhat functional group (carboxylic acid or amine) should react with a base (NaOH)?arrow_forwardMatch the description to one of the compounds E– H. a. a compound that contains a 1 ° amine and a 1 ° amide b. a compound that contains a 1 ° amine and a 2 ° amide c. a compound that contains a 2 ° amine and a 3 ° amide d. a compound that contains a 3 ° amine and a 3 ° amidearrow_forward
- amines Name and classify each compoundarrow_forwardAmide formation involves which two functional groups? A.carboxylic acid and alcohol B.alcohol and ketone C.carboxylic acid and amine D.amine and alcoholarrow_forwardWhat are the functional groups present in this antibacterial antibiotic? A. Amide, thioether, aldehyde, phenol, carboxylic acid B. Amide, thioether, ketone, amine, phenol, carboxylic acid C. Amide, thioether, ketone, phenol, carboxylic acid D. Thioether, ketone, amine, phenol, carboxylic acid A brief explanation would be highly appreciated + upvotearrow_forward
- What functional group (carboxylic acid or amine) should react with an acid (HCl)?arrow_forwardA) Name the following amine. H3C−CH2−CH2−NH−CH2−CH2−CH3 Spell out the full name of the compound. B ) Name the following amine. CH3−CH2−NH−CH2−CH2−CH3 Spell out the full name of the compound.arrow_forward1. Chief organic component of vinegar a. acetic acid b. formic acid c. benzoic acid d. butanoic acid 2. This term means without water. a. carbonyl b. hydroxyl c. anhydride d. carboyl 3. Compounds containing the cyano group. a. nitriles b. amides c. amines d. nitrates 4. General formula of a Grignard reagent. a. RCOX b. RCN c. RCOOH d. RMgX 5. Organic derivatives of ammonia, derived from replacing one, two or all three hydrogens of the ammonia. a. amide b. amine c. cyan d. nitro 6. Sulfur analogs of alcohols where the O in R-OH is replaced by sulfur. a. Thioesters b. Thiols c. Thioaldehydes d. Thioethers 7. General formula of alkanes. a. CnH2n b. CnH2n+2 c. CnH2n-2 d. R-OH 8. General formula of alkenes. a. CnH2n b. CnH2n+2 c. CnH2n-2 d. R-OH 9. General formula of alkynes. a. CnH2n b. CnH2n+2 c. CnH2n-2 d. R-OH 10. Which is soluble in water? a. methanol b. ethanol c. propanol d. all of the above 11. Which substance will have the highest boiling point? a. methanol b.…arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





