
Concept explainers
The zinc-air battery shows much promise for electric cars because it is lightweight and rechargeable:
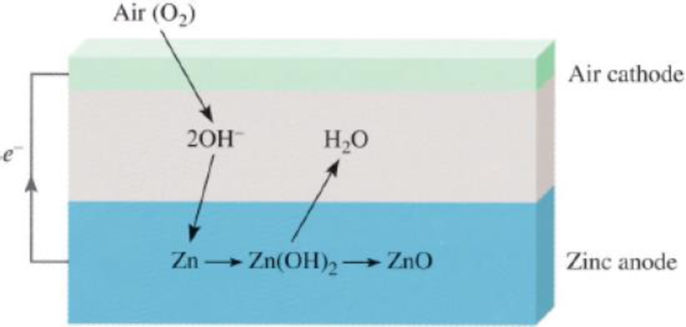
The net transformation is
(a)
Interpretation:
The
Concept Introduction:
Free energy (Gibbs free energy) is the term that is used to explain the total energy content in a thermodynamic system that can be converted into work. The free energy is represented by the letter
Where, n is the number of moles
The relation between Gibbs free energy and cell potential: The amount of energy in a system that can be converted into useful energy is defined as free energy in thermodynamics.
Free energy and the cell potential is related by the given equation.
Where,
Nernst equation is one of the important equations in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
At room temperature
Ideal gas equation is an equation that is describing the state of a imaginary ideal gas.
Where,
Answer to Problem 18.124QP
The half cell reactions of the given cell,
The standard
Explanation of Solution
To record the given data
To write the half cell reactions and overall reaction
The half cell reactions are,
Overall reaction,
To find the
The of
To find the
Using the value of free energy and the number of electrons transferred the
On rearranging the equation we get,
(b)
Interpretation:
The
Concept Introduction:
Free energy (Gibbs free energy) is the term that is used to explain the total energy content in a thermodynamic system that can be converted into work. The free energy is represented by the letter
Where, n is the number of moles
The relation between Gibbs free energy and cell potential: The amount of energy in a system that can be converted into useful energy is defined as free energy in thermodynamics.
Free energy and the cell potential is related by the given equation.
Where,
Nernst equation is one of the important equations in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
At room temperature
Ideal gas equation is an equation that is describing the state of a imaginary ideal gas.
Where,
Answer to Problem 18.124QP
The
Explanation of Solution
To record the given data
Partial pressure of oxygen
To calculate the
The
(c)
Interpretation:
The
Concept Introduction:
Free energy (Gibbs free energy) is the term that is used to explain the total energy content in a thermodynamic system that can be converted into work. The free energy is represented by the letter
Where, n is the number of moles
The relation between Gibbs free energy and cell potential: The amount of energy in a system that can be converted into useful energy is defined as free energy in thermodynamics.
Free energy and the cell potential is related by the given equation.
Where,
Nernst equation is one of the important equations in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
At room temperature
Ideal gas equation is an equation that is describing the state of a imaginary ideal gas.
Where,
Answer to Problem 18.124QP
The energy density of the zinc electrode is found to be
Explanation of Solution
To record the given data
Amount of zinc
Molecular weight of zinc
To calculate the number of moles of zinc
Number of moles of zinc in
To calculate the energy density of zinc electrode
Free energy is the maximum amount of energy in the system that can be converted into useful work. Energy density can be obtained by multiplying the free energy value with the number of moles of zinc.
(d)
Interpretation:
The
Concept Introduction:
Free energy (Gibbs free energy) is the term that is used to explain the total energy content in a thermodynamic system that can be converted into work. The free energy is represented by the letter
Where, n is the number of moles
The relation between Gibbs free energy and cell potential: The amount of energy in a system that can be converted into useful energy is defined as free energy in thermodynamics.
Free energy and the cell potential is related by the given equation.
Where,
Nernst equation is one of the important equations in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
At room temperature
Ideal gas equation is an equation that is describing the state of a imaginary ideal gas.
Where,
Answer to Problem 18.124QP
The amount of air supplied to the battery in each second is found to be
Explanation of Solution
To record the given data
Amount of current derived from the cell
To calculate the number of moles of electrons required for producing given amount of charge
Charge produced and the numbers of moles of electrons transferred are related by the following equation.
The number of moles of electrons transferred,
To calculate the number of moles of oxygen gas reduced by
From the equation for the cell reaction we have seen that
To calculate the volume of oxygen when the partial pressure is
The volume of oxygen at
To calculate the volume of air required at each second.
The volume of air required at each second is found as given below.
Want to see more full solutions like this?
Chapter 18 Solutions
CHEMISTRY 3
- For each of the reactions, calculate E from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard conditions. (a) Zn(s)+Fe2+(aq)Zn2+(aq)+Fe(s) (b) AgCl(s)+Fe2+(aq)Ag(s)+Fe3+(aq)+Cl(aq) (c) Br2(l)+2Cl(aq)Cl2(g)+2Br(aq)arrow_forwardWhat is the standard cell potential you would obtain from a cell at 25C using an electrode in which Hg22+(aq) is in contact with mercury metal and an electrode in which an aluminum strip dips into a solution of Al3+(aq)?arrow_forwardFor each reaction listed, determine its standard cell potential at 25 C and whether the reaction is spontaneous at standard conditions. (a) Mn(s)+Ni2+(aq)Mn2+(aq)+Ni(s) (b) 3Cu2+(aq)+2Al(s)2Al3+(aq)+3Cu(s) (c) Na(s)+LiNO3(aq)NaNO3(aq)+Li(s) (d) Ca(NO3)2(aq)+Ba(s)Ba(NO3)2(aq)+Ca(s)arrow_forward
- Calculate the standard cell potential of the following cell at 25C. Sn(s)Sn2+(aq)I2(aq)I(aq)arrow_forwardA half-cell that consists of a copper wire in a 1.00 M Cu(NO3)2 solution is connected by a salt bridge to a solution that is 1.00 M in both Pu3+ and Pu4+, and contains an inert metal electrode. The voltage of the cell is 0.642 V, with the copper as the negative electrode. (a) Write the half-reactions and the overall equation for the spontaneous chemical reaction. (b) Use the standard potential of the copper half-reaction, with the voltage of the cell, to calculate the standard reduction potential for the plutonium half-reaction.arrow_forwardAn electrolysis experiment is performed to determine the value of the Faraday constant (number of coulombs per mole of electrons). In this experiment, 28.8 g of gold is plated out from a AuCN solution by running an electrolytic cell for two hours with a current of 2.00 A. What is the experimental value obtained for the Faraday Constant?arrow_forward
- At 298 K, the solubility product constant for solid Ba(IO3)2 is 1.5 109. Use the standard reduction potential of Ba2+(aq) to find the standard potential for the half-reaction Ba(IO3)2(s)+2eBa(s)+2IO3(aq)arrow_forwardCalculate the cell potential of a cell operating with the following reaction at 25C, in which [Cr2O32] = 0.020 M, [I] = 0.015 M, [Cr3+] = 0.40 M, and [H+] = 0.60 M. Cr2O72(aq)+6I(aq)+14H+(aq)2Cr3+(aq)+3I2(s)+7H2O(l)arrow_forwardCalculate the cell potential of a cell operating with the following reaction at 25C, in which [MnO4] = 0.010 M, [Br] = 0.010 M. [Mn2] = 0.15 M, and [H] = 1.0 M. 2MNO4(aq)+10Br(aq)+16H+(aq)2MN2(aq)+5Br2(l)+8H2O(l)arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





