
Interpretation: A voltaic cell is given. The greater density of a concentrated solution of
Concept introduction: Voltaic cell is a type of
Salt bridge, also known as porous bridge is a U-shaped hollow device which is filled with KOH or other electrolytes. It is used to maintain the electrical neutrality during the process of redox reactions in two different compartments.
To determine: An explanation for the porous bridge not being needed in the given cell.
Answer to Problem 18.1VP
Solution
An explanation for the porous bridge not being needed in the given cell has been rightfully stated.
Explanation of Solution
Explanation
In voltaic cell during the process of redox reaction electrons are generated on anode and then transfer to the cathode by the external circuit. In this way we can say that the process of oxidation occurs at the anode and the process of reduction occurs at cathode.
Oxidation is a process in which loss of electrons take place, while in reduction gain of electron take place.
The voltaic cell is given as,
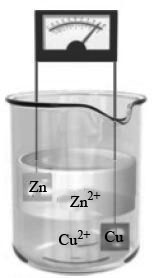
Figure 1
From the above Figure 1 it is clear that two concentrated solutions, have different densities are present in a beaker. The concentrated solution which has the greater density allows the lass concentrated solution to be layered on its top. The Figure 1 shows that one electrode is attached with zinc metal. The other electrode is attached with copper metal. Therefore, the process of oxidation occurs at zinc. Hence, electrons move from zinc metal to copper. After the loss of electrons the zinc metal converts to zinc ion. After the gain of electrons the copper metal converts to copper ion. From the above Figure 1 it is clear that the number of gained and loosed electrons is automatically balanced without interference of external source. Therefore, the electrical neutrality is automatically maintained here. Hence, in the form of reaction the above process is represented as,
Hence, it is explained that the porous bridge is not needed in the voltaic cell.
Conclusion
An explanation for the porous bridge not being needed in the given cell has been rightfully stated
Want to see more full solutions like this?
Chapter 18 Solutions
Smartwork5 Printed Access Card for Use with Chemistry: The Science in Context 5th Edition (SmartWork Access Printed Access Card)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





