
Concept explainers
(a)
Interpretation: The products formed by the Friedel-Craft alkylation of given compound with
Concept introduction: The replacement or substitution of one functional group with another different functional group in any
Answer to Problem 18.21P
The given compound does not undergo Friedel-craft alkylation reaction on reaction with
Explanation of Solution
The Friedel-craft alkylation of
The structure of given compound shows that benzene ring is substituted by
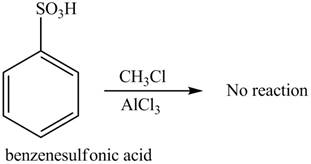
Figure 1
The given compound does not undergo Friedel-craft alkylation reaction on reaction with
(b)
Interpretation: The products formed by the Friedel-Craft alkylation of given compound with
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.21P
The given compound does not undergo Friedel-craft alkylation reaction on reaction with
Explanation of Solution
The Friedel-craft alkylation of aromatic compound with
The structure of given compound shows that benzene ring is substituted by
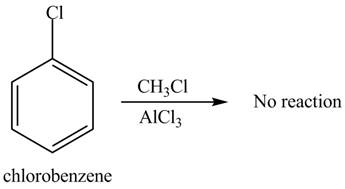
Figure 2
The given compound does not undergo Friedel-craft alkylation reaction on reaction with
(c)
Interpretation: The products formed by the Friedel-Craft alkylation of given compound with
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.21P
The given compound does not undergo Friedel-craft alkylation reaction on reaction with
Explanation of Solution
The Friedel-craft alkylation of aromatic compound with
The structure of given compound shows that benzene ring is substituted by
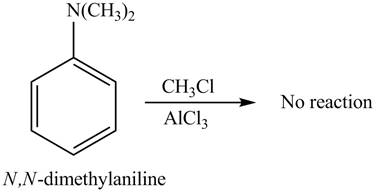
Figure 3
The given compound does not undergo Friedel-craft alkylation reaction on reaction with
(d)
Interpretation: The products formed by the Friedel-Craft alkylation of given compound with
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.21P
The products formed by the Friedel-Craft alkylation of given compound with
Explanation of Solution
The Friedel-craft alkylation of aromatic compound with
The structure of given compound shows that benzene ring is substituted by
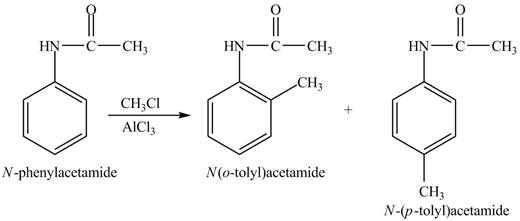
Figure 4
The products formed by the Friedel-Craft alkylation of given compound with
Want to see more full solutions like this?
Chapter 18 Solutions
ORG.CHEMISTRY CONNECT ACCESS>CUSTOM<
- Explain why toluene did or did not react witj permanganate solutionsarrow_forwarda. What is the chemical structure of biphenyl? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forwardDraw the major organic product of the reaction of carvone with KMnO4. H3O+arrow_forward
- Several diamines are building blocks for the synthesis of pharmaceuticals and agro-chemicals. Show how both 1,3-propanediamine and 1,4-butanediamine can be prepared from acrylonitrile.arrow_forwardAs we have seen in this chapter, carbon-carbon double bonds are electron-rich regions that are attacked by electrophiles (e.g., HBr); they are not attacked by nucleophiles (e.g., diethylamine). However, when the carbon-carbon double bond has a carbonyl group adjacent to it, the double bond reacts readily with nucleophiles by nucleophilic addition (Section 19.8). Account for the fact that nucleophiles add to a carbon-carbon double bond adjacent to a carbonyl group and account for the regiochemistry of the reaction.arrow_forwardMolecule Type Boiling point (°C) CH3CH2CH3 Alkane -42 CH3CHO Aldehyde +21 CH3CH2OH Alcohol +78 i. Why is the boiling point of the aldehyde greater than that of the alkane?ii. Why is the boiling point of alcohol the highest?iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forward
- 17. Which solvent between ethanol and cyclohexane has higher dipole moment? Explain.arrow_forwardWhat is the product if azobenzene react nano2/hcl and then with KCN?arrow_forwardAlkene is converted to alkyl halide by reaction with HCl . (T/F) Iodoform is used as an antiseptic. (T/F) Intermolecular bonding is strongest in alcohols than phenols. (T/F) Formation of blood red colour in Victor Meyer’s test indicated the presence of primary alcohol. (T/F) Fructose is a pentahydroxyketone . (T/F)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
