
Concept explainers
For each N-substituted benzene, predict whether the compound reacts faster than, slower than, or at a similar rate to benzene in electrophilic
a. 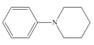 b.
b. 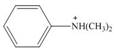 c.
c. 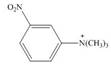 d.
d. 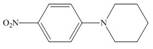
(a)
Interpretation: The given compound reacts faster than, slower than, or at equal rate to benzene in electrophilic aromatic substitution is to be predicted and the major product(s) formed by the reaction between given compound and general electrophile
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.49P
The given compound reacts faster in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
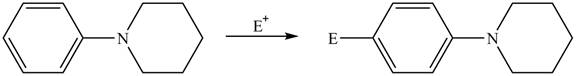
Explanation of Solution
The electron donating groups or activating groups make benzene ring more electron rich, as a result the compound reacts faster in electrophilic substitution reaction than benzene ring. On the other hand, the electron withdrawing groups or deactivating groups make benzene ring less electron rich; as a result the compound reacts slower in electrophilic substitution reaction than benzene ring.
In the given compound, benzene ring is attached to
The activating groups are ortho, para directing whereas the deactivating groups are meta directing. The major product formed by the reaction between given compound and general electrophile
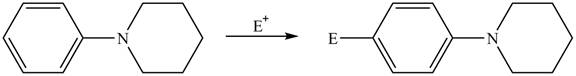
Figure 1
The given compound reacts faster in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
(b)
Interpretation: The given compound reacts faster than, slower than, or at equal rate to benzene in electrophilic aromatic substitution is to be predicted and the major product(s) formed by the reaction between given compound and general electrophile
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.49P
The given compound reacts slower in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
Explanation of Solution
The electron donating groups or activating groups make benzene ring more electron rich, as a result the compound reacts faster in electrophilic substitution reaction than benzene ring. On the other hand, the electron withdrawing groups or deactivating groups make benzene ring less electron rich; as a result the compound reacts slower in electrophilic substitution reaction than benzene ring.
In the given compound, benzene ring is attached to
The activating groups are ortho, para directing whereas the deactivating groups are meta directing. The major product formed by the reaction between given compound and general electrophile

Figure 2
The given compound reacts slower in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
(c)
Interpretation: The given compound reacts faster than, slower than, or at equal rate to benzene in electrophilic aromatic substitution is to be predicted and the major product(s) formed by the reaction between given compound and general electrophile
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.49P
The given compound reacts slower in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
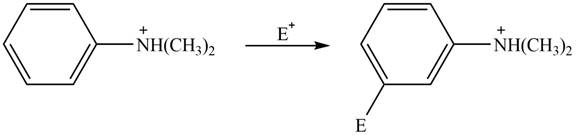
Explanation of Solution
The electron donating groups or activating groups make benzene ring more electron rich, as a result the compound reacts faster in electrophilic substitution reaction than benzene ring. On the other hand, the electron withdrawing groups or deactivating groups make benzene ring less electron rich; as a result the compound reacts slower in electrophilic substitution reaction than benzene ring.
In the given compound, benzene ring is attached to
The activating groups are ortho, para directing whereas the deactivating groups are meta directing. The major product formed by the reaction between given compound and general electrophile
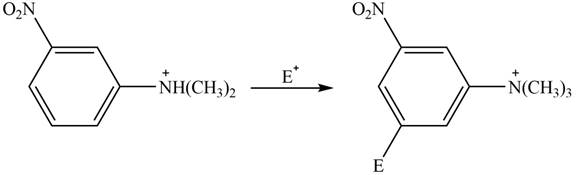
Figure 3
The given compound reacts slower in electrophilic substitution reaction than benzene ring. The major product formed by the reaction between given compound and general electrophile
(d)
Interpretation: The given compound reacts faster than, slower than, or at equal rate to benzene in electrophilic aromatic substitution is to be predicted and the major product(s) formed by the reaction between given compound and general electrophile
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.49P
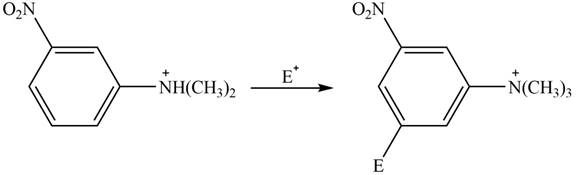
The given compound reacts at a similar rate to benzene in electrophilic substitution reaction. The major product formed by the reaction between given compound and general electrophile
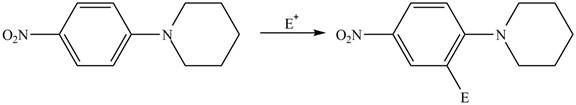
Explanation of Solution
The electron donating groups or activating groups make benzene ring more electron rich, as a result the compound reacts faster in electrophilic substitution reaction than benzene ring. On the other hand, the electron withdrawing groups or deactivating groups make benzene ring less electron rich; as a result the compound reacts slower in electrophilic substitution reaction than benzene ring.
In the given compound, benzene ring is attached to
The activating groups are ortho, para directing whereas the deactivating groups are meta directing. The major product formed by the reaction between given compound and general electrophile

Figure 4
The given compound reacts at a similar rate to benzene in electrophilic substitution reaction. The major product formed by the reaction between given compound and general electrophile
Want to see more full solutions like this?
Chapter 18 Solutions
ORG.CHEMISTRY CONNECT ACCESS>CUSTOM<
- Nicotinic acid, more commonly named niacin, is one of the B vitamins. Show how nicotinic acid can be converted to (a) ethyl nicotinate and then to (b) nicotinamide.arrow_forwardExplain the reactivity and orientation effects observed in each heterocycle.a. Pyridine is less reactive than benzene in electrophilic aromatic substitution and yields 3-substituted products.b. Pyrrole is more reactive than benzene in electrophilic aromatic substitution and yields 2-substituted products.arrow_forwardExplain the reactivity and orientation effects observed in each heterocycle. a. Pyridine is less reactive than benzene in electrophilic aromatic substitution and yields 3-substituted products. b. Pyrrole is more reactive than benzene in electrophilic aromatic substitution and yields 2-substituted products.arrow_forward
- Draw a stepwise mechanism for the sulfonation of an alkyl benzene such as A to form a substituted benzenesulfonic acid B. Treatment of B with base forms a sodium salt C that can be used as a synthetic detergent to clean away dirt (see Problem 3.22).arrow_forwardConsider the tetracyclic compound with rings labeled A–D. (a) Which ring is the most reactive in electrophilic aromatic substitution? (b) Which ring is the least reactive in electrophilic aromatic substitution?arrow_forwardA key step in the synthesis of β-vetivone, a major constituent of vetiver, a perennial grass found in tropical and subtropical regions of the world, involved the reaction of compound A and dihalide B with two equivalents of LDA to form C. Draw a stepwise mechanism for this reaction. β-Vetivone contains a spiro ring system—that is, two rings that share a single carbon atom.arrow_forward
- Consider the tetracyclic aromatic compound drawn below, with rings labeled as A, B, C, and D. (a) Which of the four rings is most reactive in electrophilic aromatic substitution? (b) Which of the four rings is least reactive in electrophilic aromatic substitution? (c) What are the major product(s) formed when this compound is treated with one equivalent of Br2?arrow_forwardDraw a stepwise mechanism for the following substitution. Explain why 2-chloropyridine reacts faster than chlorobenzene in this type of reaction.arrow_forwardFriedel-Crafts alkylations usually invariably result in a mixture of products, but Friedel acylations never do. Why? a) none of these b) alkylations require catalyst c) benzene with alkyl groups are more reactive than acylbenzenes d) the reagents used in alkylations are strongerarrow_forward
- a. Rank the following esters from most reactive to least reactive in the first slow step of a nucleophilic acyl substitution reaction (formation of thetetrahedral intermediate): b. Rank the same esters from most reactive to least reactive in the second slow step of a nucleophilic acyl substitution reaction (collapse of thetetrahedralintermediate).arrow_forwardFill in the missing reagents below.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


