
Concept explainers
(a)
Interpretation: The products formed by the treatment of given compound with
Concept introduction: The replacement or substitution of one functional group with another different functional group in any
Answer to Problem 18.37P
The products formed by the treatment of given compound with
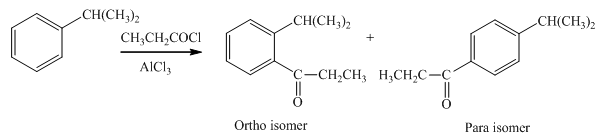
Explanation of Solution
An
In the given compound, benzene ring is attached to an electron releasing group, thus the acyl group will go to ortho and para position as shown in Figure 1.

Figure 1
The products formed by the treatment of given compound with
(b)
Interpretation: The products formed by the treatment of given compound with
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.37P
The products formed by the treatment of given compound with
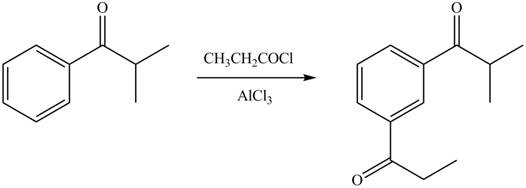
Explanation of Solution
An aromatic compound undergoes Friedel-Craft acylation on reaction with
In the given compound, benzene ring is attached to an electron withdrawing group, thus the acyl group will go to meta position as shown in Figure 2.
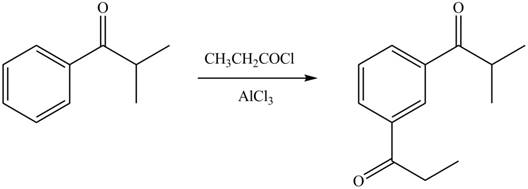
Figure 2
The products formed by the treatment of given compound with
(c)
Interpretation: The products formed by the treatment of given compound with
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.37P
No product will form by the treatment of given compound with
Explanation of Solution
An aromatic compound undergoes Friedel-Craft acylation on reaction with
In the given compound, benzene ring is attached to a strong electron releasing group. However, the Friedel-Craft acylation reaction does not occur in the presence of strong activating groups. Therefore, no product will form by the treatment of given compound with
No product will form by the treatment of given compound with
(d)
Interpretation: The products formed by the treatment of given compound with
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.37P
The products formed by the treatment of given compound with
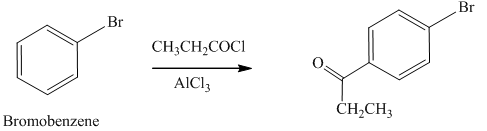
Explanation of Solution
An aromatic compound undergoes Friedel-Craft acylation on reaction with
In the given compound, benzene ring is attached to an electron withdrawing group bromine which deactivates the benzene ring and it donates electrons through resonance effect. The ortho position is an electron deficient position than para position due to negative inductive effect, thus the acyl group will go to para position as shown in Figure 4.

Figure 4
The products formed by the treatment of given compound with
(e)
Interpretation: The products formed by the treatment of given compound with
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.37P
The products formed by the treatment of given compound with
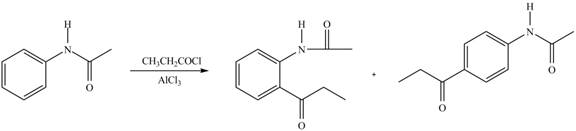
Explanation of Solution
An aromatic compound undergoes Friedel-Craft acylation on reaction with
In the given compound, benzene ring is attached to an electron releasing group, thus the acyl group will go to ortho and para position as shown in Figure 2.
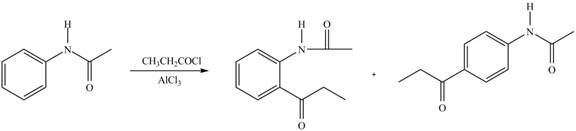
Figure 5
The products formed by the treatment of given compound with
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry - With Access (Looseleaf) (Custom)
- Draw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. d. (CH3CH2)2CHCOCl, AlCl3 j. product in (d), then NH2NH2, – OHarrow_forwardWhat product is formed when benzene is treated with each organic halide in the presence of AlCl3?arrow_forward(a) Give the IUPAC name for A and B. (b) Draw the product formed when A or B is treated with each reagent: [1] NaBH4, CH3OH; [2] CH3MgBr, then H2O; [3] Ph3P = CHOCH3; [4] CH3CH2CH2NH2, mild acid; [5] HOCH2CH2CH2OH, H+.arrow_forward
- Draw the product formed when (CH3)2CHOH is treated with each reagent. a.SOCl2, pyridine b. TsCl, pyridine c.H2SO4 d.HBr e.PBr3, then NaCN f.POCl3, pyridinearrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forwardExplain Addition of Alcohols—Acetal Formation ?arrow_forward
- a) Draw two different enol tautomers of 2-methylcyclohexanone. (b) Draw two constitutional isomers that are not tautomers, but contain a C = C and an OH group.arrow_forwardDraw the products formed when A or B is treated with each reagent. In some cases, no reaction occurs. [1] C6H5Li (excess); [2] H2Oarrow_forward(a) Give an acceptable name for compound A. (b) Draw the organic products formed when A is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
