
Concept explainers
Draw the products of each reaction.
a. 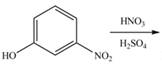 d.
d. 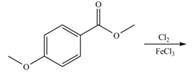
b.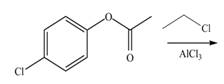 e.
e. 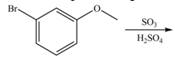
c. 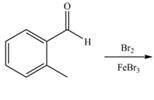 f.
f. 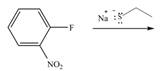
(a)
Interpretation: The products of given reaction are to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.38P
The products of given reaction are shown below:
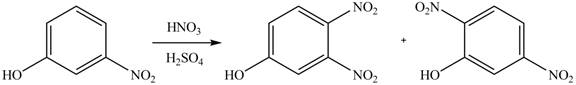
Explanation of Solution
An aromatic compound undergoes nitration on reaction with
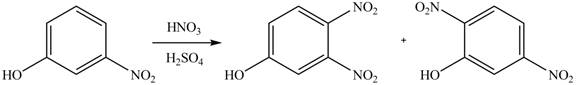
Figure 1
The products of given reaction are shown in Figure 1.
(b)
Interpretation: The products of given reaction are to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.38P
The products of given reaction are shown below:
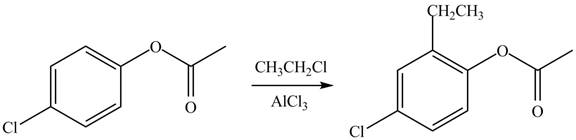
Explanation of Solution
An aromatic compound undergoes Friedel-Craft alkylation on reaction with
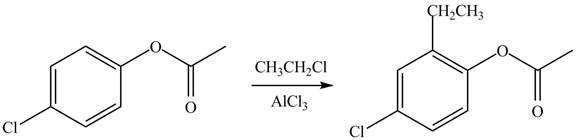
Figure 2
The products of given reaction are shown in Figure 2.
(c)
Interpretation: The products of given reaction are to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.38P
The products of given reaction are shown below:
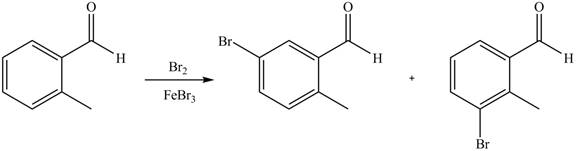
Explanation of Solution
An aromatic compound undergoes bromination on reaction with
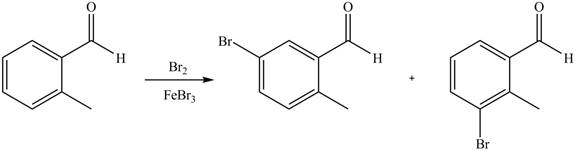
Figure 3
The products of given reaction are shown in Figure 3.
(d)
Interpretation: The products of given reaction are to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.38P
The products of given reaction are shown below:
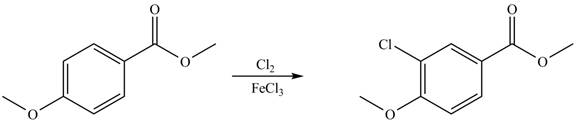
Explanation of Solution
An aromatic compound undergoes chlorination on reaction with
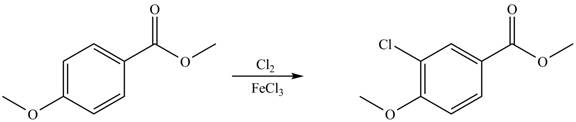
Figure 4
The products of given reaction are shown in Figure 4.
(e)
Interpretation: The products of given reaction are to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.38P
The products of given reaction are shown below:
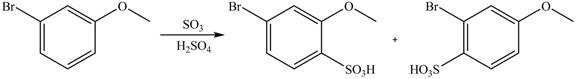
Explanation of Solution
An aromatic compound undergoes sulfonation on reaction with
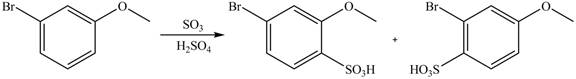
Figure 5
The products of given reaction are shown in Figure 5.
(f)
Interpretation: The products of given reaction are to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.38P
The products of given reaction are shown below:
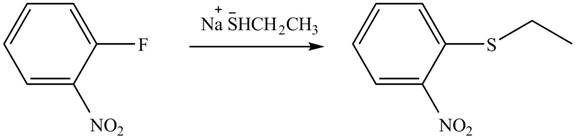
Explanation of Solution
In the given reaction,
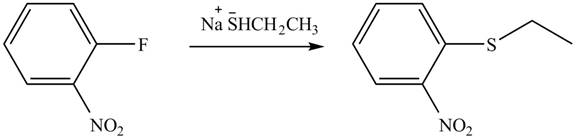
Figure 6
The products of given reaction are shown in Figure 6.
Want to see more full solutions like this?
Chapter 18 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





