
Concept explainers
Interpretation:
Classification of reaction below into oxidation and reduction has to be determined. Whether
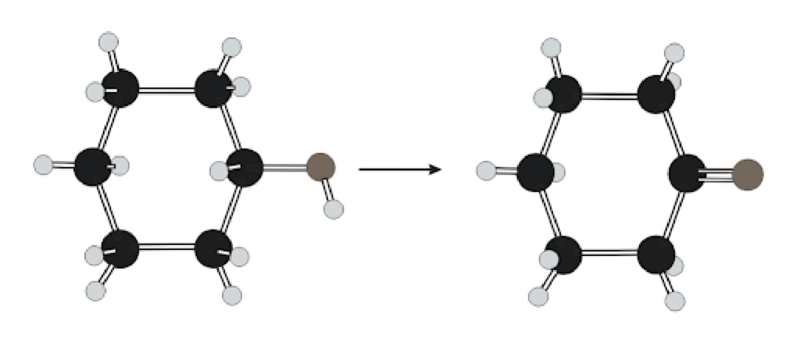
Concept Introduction:
In
Oxidizing agent reduces to complete oxidation reaction while reducing agent oxidizes to complete reduction reaction.
More convenient way to spot
Coenzyme
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Principles of General Organic & Biological Chemistry
- Label the reaction as an oxidation or reduction and give the coenzyme, NAD + or NADH, which might be used to carry out the transformation. Write the reaction using skeletal structures with curved arrow symbolism.arrow_forwardWhich of the terms hydrolysis, saponification, hydrogenation, and oxidation apply to each of the following reaction changes? More than one term may apply in a given situation. 1) Carbon-carbon double bonds are broken. 2) Fatty acids are among the products.arrow_forwardGLYCOLYSIS: 1A) Starting with glucose (in the open-chain Fisher projection), draw out the molecular structures for each step of glycolysis. For each step, include the name of the enzyme that catalyzes the reaction. 1B) What is the net reaction of glycolysis? CITRIC ACID CYCLE: 2A) Starting with acetyl-coenzymeA and oxaloacetate, draw out the molecular structures for each step of the citric acid cycle. For each step, include the name of the enzyme that catalyzes the reaction. 2B) What is the net reaction of the citric acid cycle? What happens to each product? OXIDATIVE PHOSPHORYLATION: 3A) Thoroughly explain the biological significance of NADH/H* and FADH2 and their roles in oxidative phosphorylation. 3B) Describe and diagram the general pathway of the electrons from NADH/H+ and FADH₂ to O₂ during the electron transport chain. 3C) What is "chemiosmotic coupling", and what is its relationship to ATP synthesis? 3D) During oxidative phosphorylation, what is oxidized and what is…arrow_forward
- ATP is produced during synthesis of compounds. O True O Falsearrow_forwardWrite the reaction catalyzed by catalase?arrow_forward2 A reaction is completed on oleic acid that removes its double bond and saturates its fatty acid chain to become completely saturated. What is true about the product? a) The melting point of the product will be greater than that of the starting material. Ob) The intermolecular forces between molecules of the product will be less than those between molecules of starting material. c) The number of kinks in the fatty acid chain will increase.arrow_forward
- Use the following to answer the questions below: For each of the reaction characterizations, select from the response list the type of reaction of fats and oils that will produce the indicated effect. Responses may be used more than once or need not be used at all. a) hydrogenation b) rancidity c) saponfication d) hydrolysis Produces fatty acid saltsarrow_forwardDescribe the transport and storage of fatty acids in humans with particular reference to disease states.arrow_forwardWhat kinds of substrates do the carboxylating coenzymes work on?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





