
Concept explainers
(a)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
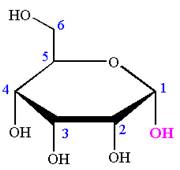
Explanation of Solution
The given molecule is
The structure of
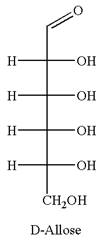
The name
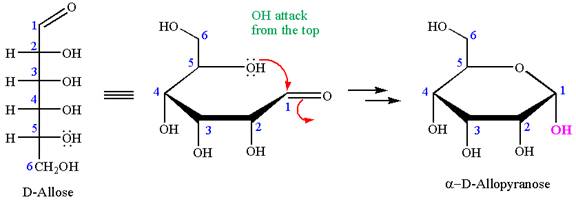
The Haworth projection of
(b)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
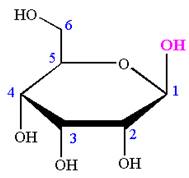
Explanation of Solution
The given molecule is
The structure of
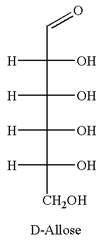
The name
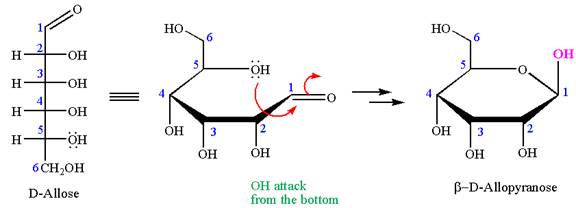
The Haworth projection of
(c)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
Explanation of Solution
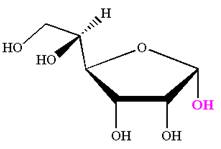
The given molecule is
The structure of
The name
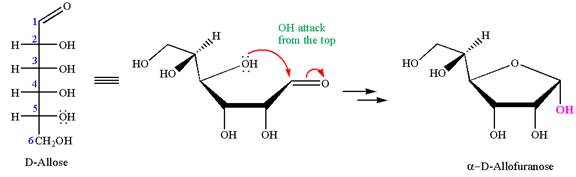
The Haworth projection of
(d)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
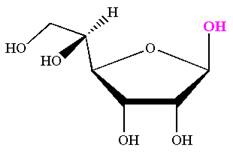
Explanation of Solution
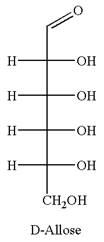
The given molecule is
The structure of

The name
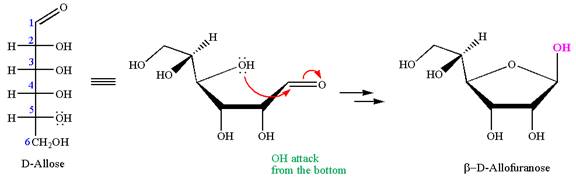
The Haworth projection of
Want to see more full solutions like this?
Chapter 18 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





