
To calculate:
a) The radius of the
b) The density of simple cubic
c) The percent empty space in a unit cell of
Answer to Problem 18.94QA
Solution:
a) The radius of the
b)
c) Total volume of empty space
Explanation of Solution
1) Concept:
a)
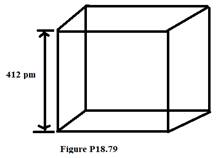
b) Volume of the unit cell is the cube of the edge length. Using the given value of edge length, we can calculate its volume. Simple cubic has one atom per unit cell. So, using molar mass of
c) The packing efficiency is calculated using volumes of one
2) Formula:
i) bcc:
ii) Simple cubic:
iii) Volume,
iv)
v)
vi)
3) Given:
i)
ii)
4) Calculations:
a) Calculate the radius of
b) Calculate the edge length in cm from
Calculate the volume of simple cubic
Mass of
c) Volume of
Volume of
So, the total volume of
Calculate the volume of simple cubic
Conclusion:
We calculate the radius of the cation from the relation between the edge length and radius. The density of the simple cube is calculated from the edge length. Packing efficiency is calculated from the volume of atoms and volume of unit cell.
Want to see more full solutions like this?
Chapter 18 Solutions
CHEMISTRY ATOM FOCUSED EBK W/ A.C. >I<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





