
(a)
Interpretation:
From the given compounds from A to D, a compound containing a primary amine and a primary alcohol needs to be identified.
Concept Introduction:
Amine − is an organic N compounds formed by replacing one or more H atoms of
Alcohol- is an organic compound contains the group of
Answer to Problem 29P
Structure C.
Explanation of Solution
Amines are formed by replacing one or more hydrogen atoms of ammonia with alkyl groups.
Furthermore,
In the below stick and ball structure, C atoms are representing in black color, H atoms in white color, N atoms in blue color and O atoms in red color. Stick and ball structure can be simplified as below;
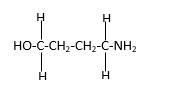
Bond that is indicated in red color is the only one
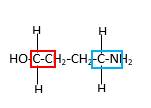
Hence, structure C is a compound that contains a
(b)
Interpretation:
From the given compounds from A to D, a compound containing a secondary amine and a secondary alcohol needs to be identified.
Concept Introduction:
Amine − is an organic N compounds formed by replacing one or more H atoms of
Alcohol- is an organic compound contains the group of
Answer to Problem 29P
Structure D.
Explanation of Solution
Amines are formed by replacing one or more hydrogen atoms of ammonia with alkyl groups.
Furthermore,
In the below stick and ball structure, C atoms are representing in black color, H atoms in white color, N atoms in blue color and O atoms in red color. Stick and ball structure can be simplified as below;
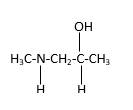
Bonds that are indicated in red color are the two
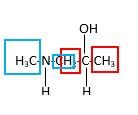
Hence, structure D is a compound that contains a
(c)
Interpretation:
From the given compounds from A to D, a compound containing a primary amine and a tertiary alcohol needs to be identified.
Concept Introduction:
Amine − is an organic N compounds formed by replacing one or more H atoms of
Alcohol- is an organic compound contains the group of
Answer to Problem 29P
Structure B.
Explanation of Solution
Amines are formed by replacing one or more hydrogen atoms of ammonia with alkyl groups.
Furthermore,
In the below stick and ball structure, C atoms are representing in black color, H atoms in white color, N atoms in blue color and O atoms in red color. Stick and ball structure can be simplified as below;
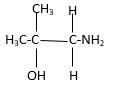
Bonds that are indicated in red color are the three
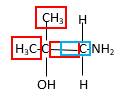
Hence, structure B is a compound that contains a
(d)
Interpretation:
From the given compounds from A to D, a compound containing a tertiary amine and a primary alcohol needs to be identified.
Concept Introduction:
Amine − is an organic N compounds formed by replacing one or more H atoms of
Alcohol- is an organic compound contains the group of
Answer to Problem 29P
Structure A.
Explanation of Solution
Amines are formed by replacing one or more hydrogen atoms of ammonia with alkyl groups.
Furthermore,
In the below stick and ball structure, C atoms are representing in black color, H atoms in white color, N atoms in blue color and O atoms in red color. Stick and ball structure can be simplified as below;
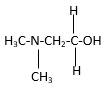
Bond that is indicated in red color is the only one
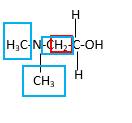
Hence, structure A is a compound that contains a
Want to see more full solutions like this?
Chapter 18 Solutions
GENERAL, ORGANIC & BIOLOGICAL CHEMISTRY
- Which type of amine is phentermine? a) a primary aliphatic amine b) a primary aromatic amine c) a tertiary aliphatic amine d) a tertiary aromatic aminearrow_forwardA) Name the following amine. H3C−CH2−CH2−NH−CH2−CH2−CH3 Spell out the full name of the compound. B ) Name the following amine. CH3−CH2−NH−CH2−CH2−CH3 Spell out the full name of the compound.arrow_forwardWhich type of amine is (s)-methamphetamine? a) a primary aliphatic amine b) a primary aromatic amine c) a secondary aliphatic amine d) a secondary aromatic aminearrow_forward
- Explain in details , the reactions of amines (hinsberg test)arrow_forward1. Which of the following DOES NOT belong to the group? * A. Amine B. Nitrile C. Amide D. Acyl Halide 2. What is the IUPAC name of the compound? (Please refer to the first image attached.) A. 4,4-Dibromobutane B. 1,1-Dibromobutane C. 1-Butyl-2,2-dibromine D. Butyl-2,2-Dibromobutane E. None of the choices 3. What is the IUPAC name of the compound (Please refer to the second image attached.) A. 1-Cyclohexyl-1-cyclopropylcyclohexane B. 1-Cyclopropyl-1-cyclohexylcyclohexane C. Cycloropyl-1,1-dicyclohexane D. 1,2-Dicyclohexyl-1-cyclopropane E. None of the choicesarrow_forwardDraw a structural formula for each amine and amine derivative. Q.) trans-2-Aminocyclohexanolarrow_forward
- A carbonyl group and a hydroxyl group bonded to the same carbon atom must be present in a carboxylic acid. A secondary alcohol is produced when a carboxylic acid is reduced. Both statements are true Both statements are false Only the first statement is true Only the second statement is true 2. Which of the following statements is TRUE? I. Long-chain amines are insoluble in water. II. Amines can be dissolved in an aqueous base. III. Amines can be dissolved in aqueous acid. I and II only I only I, II, and III I and III onlyarrow_forwardN-p-hydroxyphenylethanamide is commonly known as a. acetaminophen b. acetamide c. acetanilide d. formamide High molar mass amines have __________ odor. a.strong ammoniacal b.fruity c.fishy d.obnoxious Trimethyl amine has _________ odor. a.obnoxious b.fishy c. ammoniacal d. fruityarrow_forward1. What allows some organic compounds to be soluble in water? 2. If two solutions are insoluble with each other, describe how a mixture of the two solutions would appear. 3. Explain the saying “like dissolves like” 4. What is the trend in solubility for alcohols in water as the number of carbons in the parent chain increases. Explain this trend? 5. Which of the following would be the most soluble and least soluble in water Primary amines, secondary amines, tertiary amines. Explain your reasoning. 6. Would you expect an alcohol with a 20-carbon parent chain to be soluble or insoluble in oil? Why?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

