
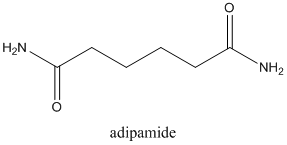
(a)
Interpretation:
The carboxylic acid and
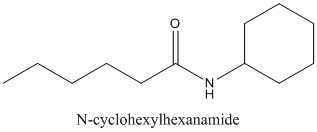
Concept Introduction:
Amides are formed by a reaction with a carboxylic acid and an amine which are the functional groups. This results in the formation of amide bond, in which OH group of carboxylic acid reacts with one of the H in amine group and CO-NH bond is formed. This bond is known as an amide bond.
The mechanism for formation of an amide is as follows taking example of propanoic acid and ethylamine:
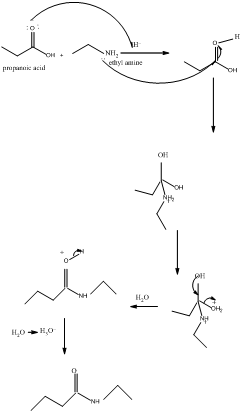
The hydrolysis of amide so formed can give back the carboxylic acid and amine.
(b)
Interpretation:
The carboxylic acid and amine or ammonia needs to be identified from which each amide be synthesized.
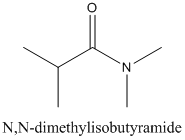
Concept Introduction:
Amides are formed by a reaction with a carboxylic acid and an amine which are the functional groups. This results in the formation of amide bond, in which OH group of carboxylic acid reacts with one of the H in amine group and CO-NH bond is formed. This bond is known as an amide bond.
The mechanism for formation of an amide is as follows taking example of propanoic acid and ethylamine:
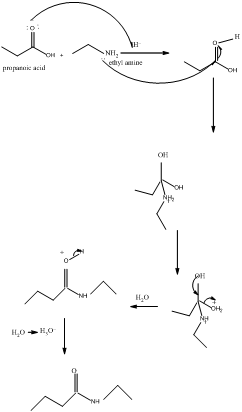
The hydrolysis of amide so formed can give back the carboxylic acid and amine.
(c)
Interpretation:
The carboxylic acid and amine or ammonia needs to be identified from which each amide be synthesized.
Concept Introduction:
Amides are formed by a reaction with a carboxylic acid and an amine which are the functional groups. This results in the formation of amide bond, in which OH group of carboxylic acid reacts with one of the H in amine group and CO-NH bond is formed. This bond is known as an amide bond.
The mechanism for formation of an amide is as follows taking example of propanoic acid and ethylamine:
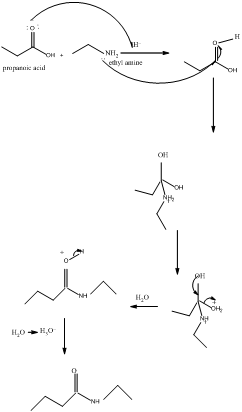
The hydrolysis of amide so formed can give back the carboxylic acid and amine.
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Introduction To General, Organic, And Biochemistry
- 3 Compare the basicity of amines with other common bases, and explain how theirbasicity varies with hybridization and aromaticity.arrow_forwardThe pKa of acetamide (CH3CONH2) is 16. Draw the structure for its conjugate base and explain why acetamide is less acidic than CH3COOH.arrow_forwardAmide hydrolysis in basic conditions forms A. a carboxylic acid and an amine B. a carboxylate salt and an amine 3. an ester and an amine 4. a carboxylic acid and an amine saltarrow_forward
- Account for the following :(i) Primary amines (R-NH2) have higher boiling point than tertiary amines (R3N).(ii) Aniline does not undergo Friedel – Crafts reaction.(iii) (CH3)2NH is more basic than (CH3)3N in an aqueous solution.arrow_forward2. Enumerate 3 biologically important amines and their use.arrow_forwardgive the results of the following compounds after reaction with nitrous acid test a. primary aliphatic amine b. secondary aliphatic amine c. tertiary aliphatic amine d. primary aromatic aminearrow_forward
- Each pair of compounds below can be separatedusing acid/base extraction. Provide the common acid or base that could be used to effect the separation. Amines can be converted into ammonium salts using acid, but neutral amines are very weak acids (pKa~ 35)and thus cannot be extracted by an aqueous base.arrow_forwardwhat is the structure of o-methylphenoxide anion and what is its pKa?arrow_forwardWhat 1° amine and carbonyl compound are needed to prepare attached imine?arrow_forward
- What carboxylic acid and amine are needed to synthesize the pain reliever phenacetin? Phenacetin was once a component of the over-the-counter pain reliever APC (aspirin, phenacetin, caffeine), but it is no longer used because of its kidney toxicity.arrow_forwardPlace the binders below in descending order of acidity (pi) and justify your choice: CH3CN; (C2H5)2O; PCl3; As(C6H5)3; (C2H5)3Narrow_forwardExplain the observed difference in the pKa values of the conjugate acids of amines A and B.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

