
Concept explainers
(a)
Interpretation:
Concept introduction:
Solubility:
Solubility is defined as the maximum amount of the solute that can be dissolved in the solvent at equilibrium.
Solubility product constant:
Solubility product constant is defined for equilibrium between solids and its respective ions in the solution. Generally, solubility product refers only to insoluble or slightly soluble ionic substances that make equilibrium in water.
It is defined as the product of concentration of ions of a sparingly soluble salt in its saturated solution at
This value indicates the degree of dissociation of a compound in water. More the value of
Considering an equilibrium of salt
Formation constant:
A stability constant or formation constant is an equilibrium constant for the formation of a complex ion in the solution and it measures the strength of interaction between the reactants that forms the complex.
(a)
Answer to Problem 19.148P
It is proved that
Explanation of Solution
Given that the equations are,
Subtracting the above equation (1) from equation (2),
Substituting the value of
Therefore it is proved that
(b)
Interpretation:
Value of
Concept introduction:
Solubility:
Solubility is defined as the maximum amount of the solute that can be dissolved in the solvent at equilibrium.
Solubility product constant:
Solubility product constant is defined for equilibrium between solids and its respective ions in the solution. Generally, solubility product refers only to insoluble or slightly soluble ionic substances that make equilibrium in water.
It is defined as the product of concentration of ions of a sparingly soluble salt in its saturated solution at
This value indicates the degree of dissociation of a compound in water. More the value of
Considering an equilibrium of salt
Formation constant:
A stability constant or formation constant is an equilibrium constant for the formation of a complex ion in the solution and it measures the strength of interaction between the reactants that forms the complex.
(b)
Answer to Problem 19.148P
The value of
Explanation of Solution
Given that,
Now the condition is given that
From the formation constant,
Substituting the value of
Now according to given condition,
Therefore
(c)
Interpretation:
Plot of solubility of
Concept introduction:
Solubility:
Solubility is defined as the maximum amount of the solute that can be dissolved in the solvent at equilibrium.
Solubility product constant:
Solubility product constant is defined for equilibrium between solids and its respective ions in the solution. Generally, solubility product refers only to insoluble or slightly soluble ionic substances that make equilibrium in water.
It is defined as the product of concentration of ions of a sparingly soluble salt in its saturated solution at
This value indicates the degree of dissociation of a compound in water. More the value of
Considering an equilibrium of salt
Le Chatelier’s principle:
When a system in equilibrium then is subjected to any external disturbance like change of pressure, volume, temperature etc… Then the system acts in a way to prevent that change. This is called Le-Chatelier’s principle.
(c)
Explanation of Solution
Given that,
When the concentration of
As the
After that if
The graph is given below,
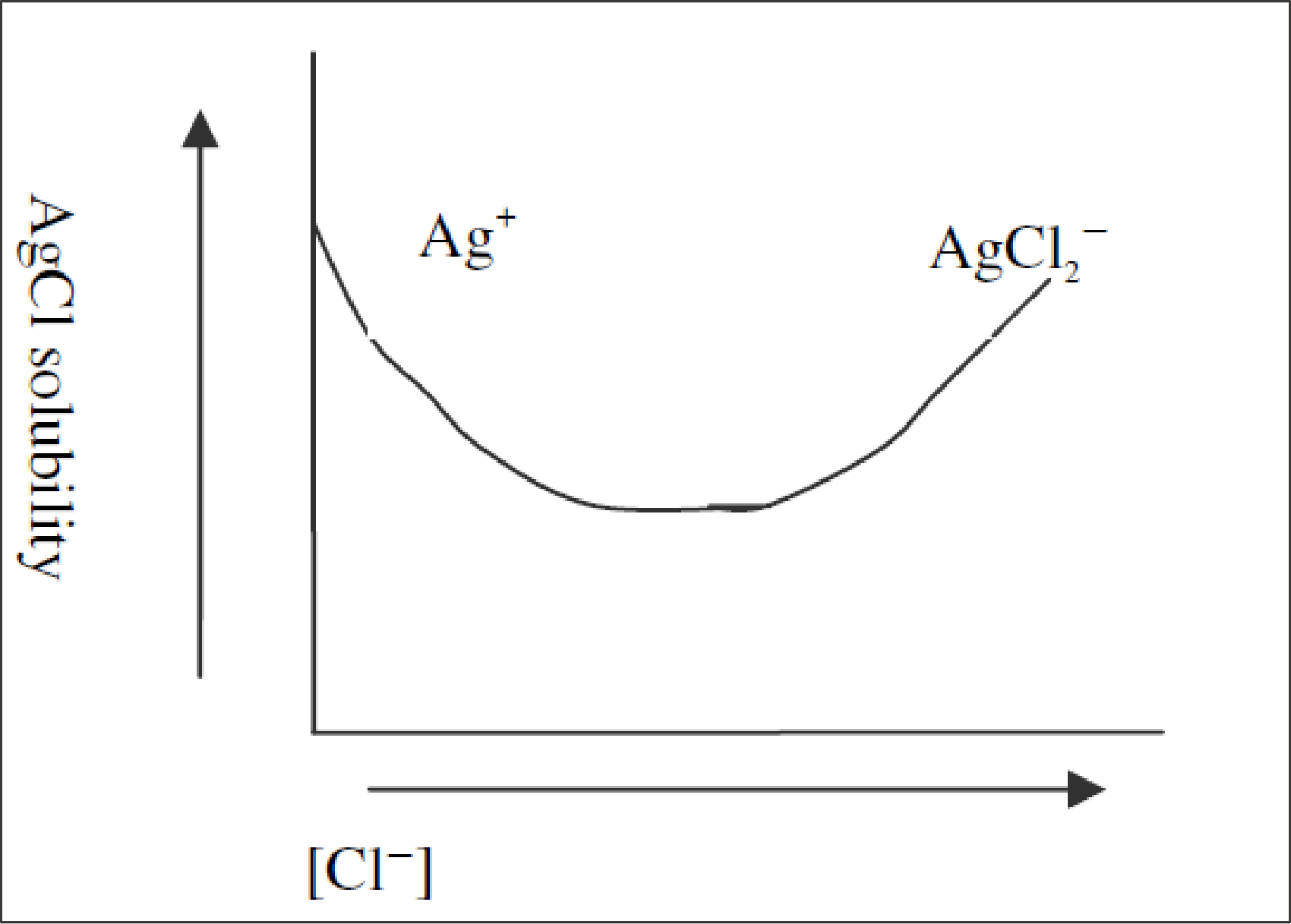
Figure.1
(d)
Interpretation:
The solubility of
Concept introduction:
Solubility:
Solubility is defined as the maximum amount of the solute that can be dissolved in the solvent at equilibrium.
Solubility product constant:
Solubility product constant is defined for equilibrium between solids and its respective ions in the solution. Generally, solubility product refers only to insoluble or slightly soluble ionic substances that make equilibrium in water.
It is defined as the product of concentration of ions of a sparingly soluble salt in its saturated solution at
This value indicates the degree of dissociation of a compound in water. More the value of
Considering equilibrium of salt
Formation constant:
A stability constant or formation constant is an equilibrium constant for the formation of a complex ion in the solution and it measures the strength of interaction between the reactants that forms the complex.
(d)
Answer to Problem 19.148P
The solubility of
Explanation of Solution
The condition given
From that it can be concluded that the value of
The condition is that solubility of
It is already found that,
Hence the solubility of
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





