
Concept explainers
Which compound in each pair has the lower
a. 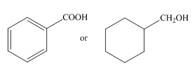 c.
c. 
b.
(a)
Interpretation: The compound in the given pair that has lower
Concept introduction: An acid is the substance that has the ability to donate a proton in the chemical reaction and base is a substance that accepts a proton. The species that do not possess hydrogen ions and formed from an acid are known as the conjugate base. The major factors that contribute to the acidic strength are inductive effect and polarity.
Answer to Problem 19.37P
The compound benzoic acid has lower
Explanation of Solution
The given compounds are,
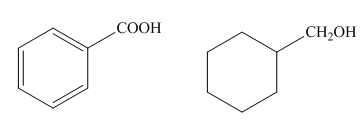
Figure.1
The strength of an acid depends upon the stability of conjugate base. Stable is the conjugate base, higher is the acidity of acid. The anion formed in benzoic acid is resonance stabilized and the negative charge is delocalized on two highly electronegative oxygen atom. Thus, the conjugate base formed by the benzoic acid is more stable than that formed by cyclohexyl methanol. The compounds which have lower
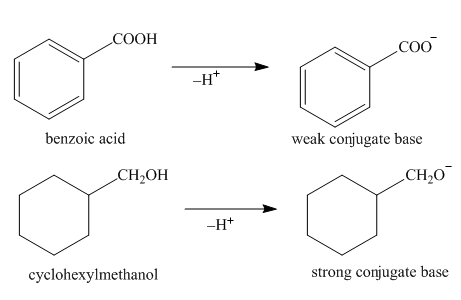
Figure 2
The resonance stabilization of benzoic acid is shown below.
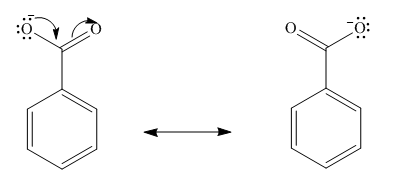
Figure 3
Therefore, the compound benzoic acid has lower
The compound benzoic acid has lower
(b)
Interpretation: The compound in the given pair that has lower
Concept introduction: An acid is the substance that has the ability to donate a proton in the chemical reaction and base is a substance that accepts a proton. The species that do not possess hydrogen ions and formed from an acid are known as the conjugate base. The major factors that contribute to the acidic strength are inductive effect and polarity.
Answer to Problem 19.37P
The compound
Explanation of Solution
The given compounds are
The electron withdrawing groups increase the acidic strength of the compound. Fluorine is more electronegative than chlorine. Due to this, the acidic strength of
The
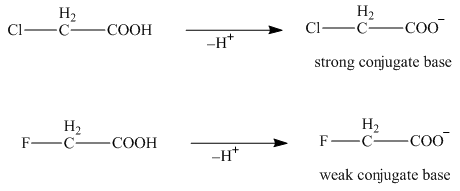
Figure 4
Therefore, the compound
The compound
(c)
Interpretation: The compound in the given pair that has lower
Concept introduction: An acid is the substance that has the ability to donate a proton in the chemical reaction and base is a substance that accepts a proton. The species that do not possess hydrogen ions and formed from an acid are known as the conjugate base. The major factors that contribute to the acidic strength are inductive effect and polarity.
Answer to Problem 19.37P
The compound
Explanation of Solution
The given compounds are shown below.
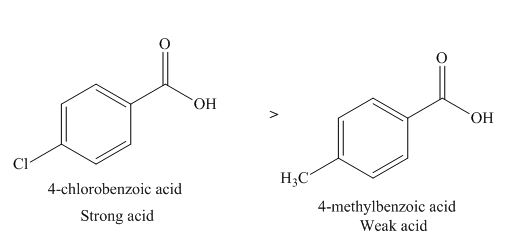
Figure 5
The electron withdrawing groups increase the acidic strength of the compound. Chlorine is electron withdrawing group, whereas methyl is electron donating group. Due to this, the acidic strength of
The
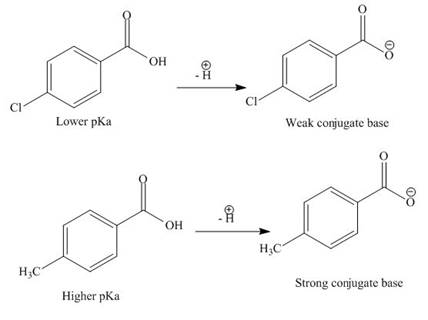
Figure 6
Therefore, the compound
The compound
(d)
Interpretation: The compound in the given pair that has lower
Concept introduction: An acid is the substance that has the ability to donate a proton in the chemical reaction and base is a substance that accepts a proton. The species that do not possess hydrogen ions and formed from an acid are known as conjugate base. The major factors that contribute to the acidic strength are
Answer to Problem 19.37P
The compound
Explanation of Solution
The given compounds are shown below.
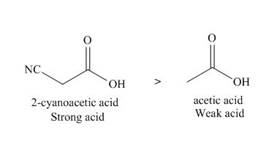
Figure 7
The electron withdrawing groups increase the acidic strength of the compound. Cyano is an electron withdrawing group. Due to this, the acidic strength of
The
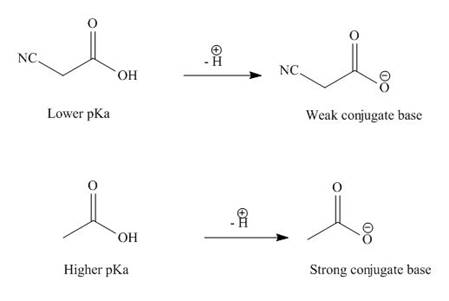
Figure 8
Therefore, the compound
The compound
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry -Study Guide / Solution Manual (Custom)
- Which base, CH3NH2 or (CH3)2NH, is the stronger base? Which conjugate acid, (CH3)2NH2+ or (CH3)2NH3+, is the stronger acid?arrow_forward1) Which of the following is a nonredox reaction? 2PbSO4(s)+heat—>2PbSO3(s)+O2(g) 2Li(s)+Mg(NO3)2(aq)—->2LiNO3(aq)+Mg(s) S8(s)+8O2(g)—->8SO2(g) None H2SO4(aq)+2NH4OH(aq)—->2H2O(l)+(NH4)2SO4(aq) 2) What is the conjugate base in reaction: HSO4^-(aq)+H2O(l) H2SO4(aq)+OH^-(aq) A) HSO4^- B) H2O C) OH^- D) H2SO4 E) nonearrow_forwarda.Draw the conjugate acid of each base: NH3, Cl−, (CH3)2C=O b. Draw the conjugate base of each acid: HBr, HSO4−, CH3OH.arrow_forward
- Which is the stronger base for each pair?arrow_forwardPick the stronger base from each pair.a. ClO4- or ClO2-b. Cl- or H2O c. CN- or ClOExercisesarrow_forwardBF3(solv) + NaF(solv) Na+(solv) + BF4-(solv) is best described as reaction. (A) an Arrhenius acid/base(B) a Brønsted-Lowry acid/base (C) a Lewis acid/base (D) all of the above (E) none of the above (F) A & B (G) A&C(H) B&C(I) World Seriesarrow_forward
- Predict the stronger acid in each pair: (a) HNO3 or HNO2;(b) H2S or H2O; (c) H2SO4 or H2SeO4; (d) CH3COOH or CCl3COOH.arrow_forwardWhat is the pOH of:(a) a 0.0100 F solution of phtalic acid?(b) a 0.0100 F solution of monopotassium phtalate? (c) a 0.0100 F solution of dipotassium phtalate?if pKa1 = 2.950 and pKa2 = 5.408 for phtalic acidarrow_forwardDraw the products formed when 2−propanol [(CH3)2CHOH], the main ingredient of rubbing alcohol, is treated with H2SO4 conjugate acid and conjugate base can I please have a step by step answer with why you do each step .arrow_forward
- H2S+H2O H3O+ + HS- Which specie is the conjugate acid?arrow_forwardExplain why the C – Ha bond is much more acidic than the C – Hb bond in pentan-2-one.arrow_forwardConsider the following reaction: HBr + −C N Br− + HC N a. What is the acid on the left side of the equation? b. What is the base on the left side of the equation? c. What is the conjugate base of the acid on the left? d. What is the conjugate acid of the base on the left? e. What is the acid on the right side of the equation? f. What is the base on the right side of the quation? g. What is the conjugate base of the acid on the right? h. What is the conjugate acid of the base on the right?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
