
Concept explainers
(a)
Interpretation:
The number of unpaired electrons expected in
(a)
Explanation of Solution
Ground state electronic configuration of
Ground state electronic configuration of
The energy level diagram of
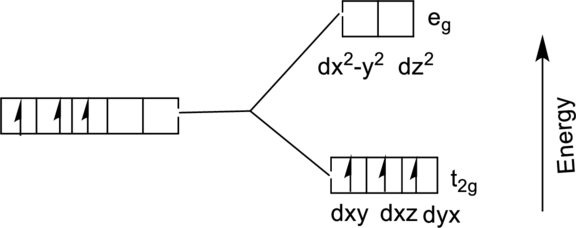
From the above diagram, the number of unpaired electrons expected in
(b)
Interpretation:
The number of unpaired electrons expected in
(b)
Explanation of Solution
Ground state electronic configuration of
Ground state electronic configuration of
The energy level diagram of
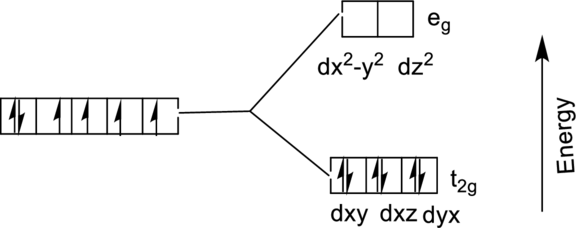
From the above diagram, the number of unpaired electrons expected in
(c)
Interpretation:
The number of unpaired electrons expected in
(c)
Explanation of Solution
Ground state electronic configuration of
Ground state electronic configuration of
The energy level diagram of
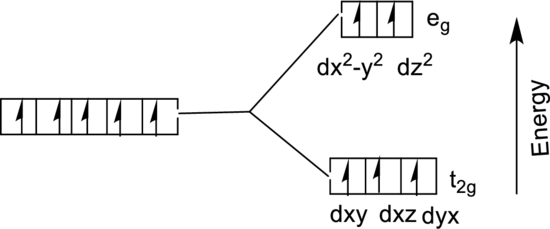
From the above diagram, the number of unpaired electrons expected in
(d)
Interpretation:
The number of unpaired electrons expected in
(d)
Explanation of Solution
Ground state electronic configuration of
Ground state electronic configuration of
The energy level diagram of
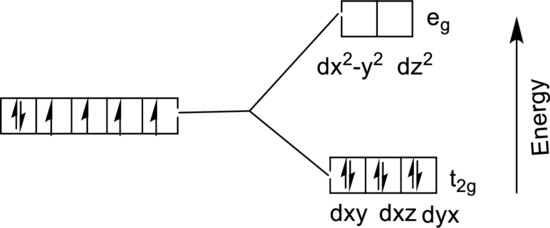
From the above diagram, the number of unpaired electrons expected in
(e)
Interpretation:
The number of unpaired electrons expected in
(e)
Explanation of Solution
Ground state electronic configuration of
Ground state electronic configuration of
The energy level diagram of
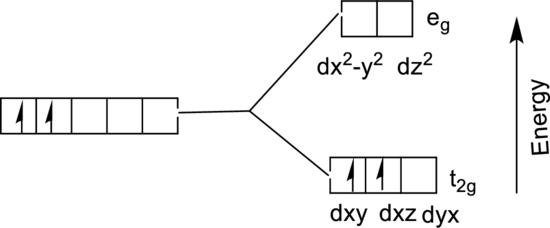
From the above diagram, the number of unpaired electrons expected in
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





