
(a)
Interpretation:
The Major organic product of the given reaction is to be determined.
Concept introduction:
The palladium catalyzed reactions are namely Suzuki coupling and Heck coupling which formed new
Answer to Problem 19.61P
The major product of given Suzuki reaction of
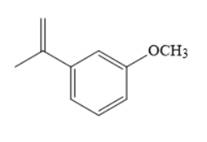
Explanation of Solution
The Suzuki coupling reaction forms a new
Therefore, the overall Suzuki reaction is
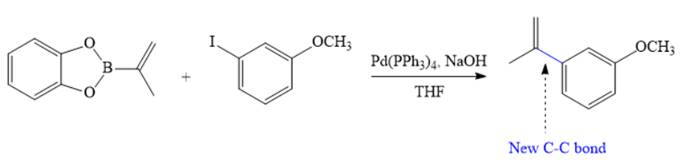
Suzuki reaction, involving possible
(b)
Interpretation:
The Major organic product of the given reaction is to be determined.
Concept introduction:
The palladium catalyzed reactions are namely Suzuki coupling and Heck coupling which formed new
Answer to Problem 19.61P
The major product of the given Suzuki reaction of
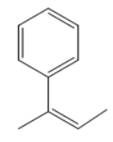
Explanation of Solution
The Suzuki coupling reaction forms a new
Therefore, the overall Suzuki reaction is
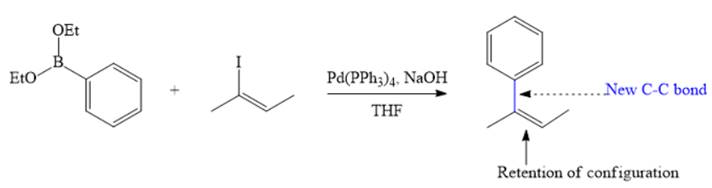
Suzuki reaction involving possible E/Z isomerism usually proceeds with retention of configuration of C=C double bond.
(c)
Interpretation:
The Major organic product of the given reaction is to be determined.
Concept introduction:
The palladium catalyzed reactions are namely Suzuki coupling and Heck coupling which formed new
Answer to Problem 19.61P
The major product of given Heck reaction of
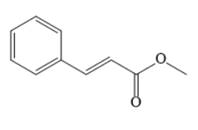
Explanation of Solution
In the given Heck reaction, a new
Therefore, the overall Heck reaction is
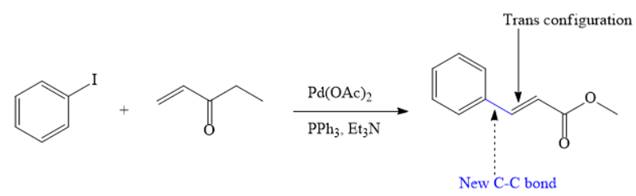
If the
(d)
Interpretation:
The Major organic product of the given reaction is to be determined.
Concept introduction:
The palladium catalyzed reactions are namely Suzuki coupling and Heck coupling which formed new
Answer to Problem 19.61P
The major product of given Heck reaction of
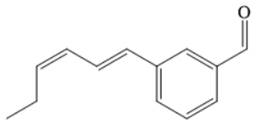
Explanation of Solution
The
Therefore, the overall Heck reaction is
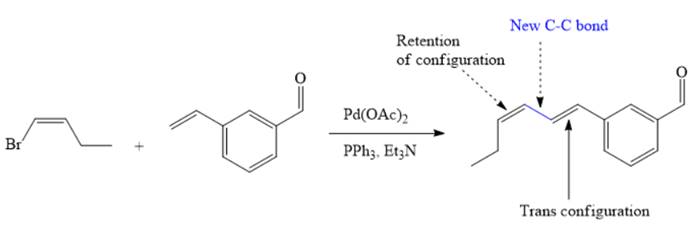
If the
Want to see more full solutions like this?
Chapter 19 Solutions
ORGANIC CHEM BUNDLE
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





