
Organic Chemistry&mod Mstg Etx Vp Ac Pkg
1st Edition
ISBN: 9780134466729
Author: Bruice
Publisher: Pearson Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 43P
Organic chemists work with tetraphenylporphyrins rather than with porphyrins because tetraphenylporphyrins are much more resistant to air oxidation, Tetraphenylporphyrin can be prepared by the reaction of benzaldehyde with pyrrole. Please a mechanis, for the formation of the ring system shown here:
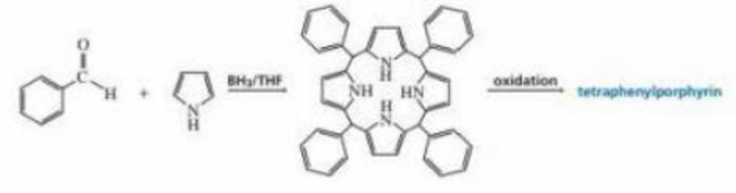
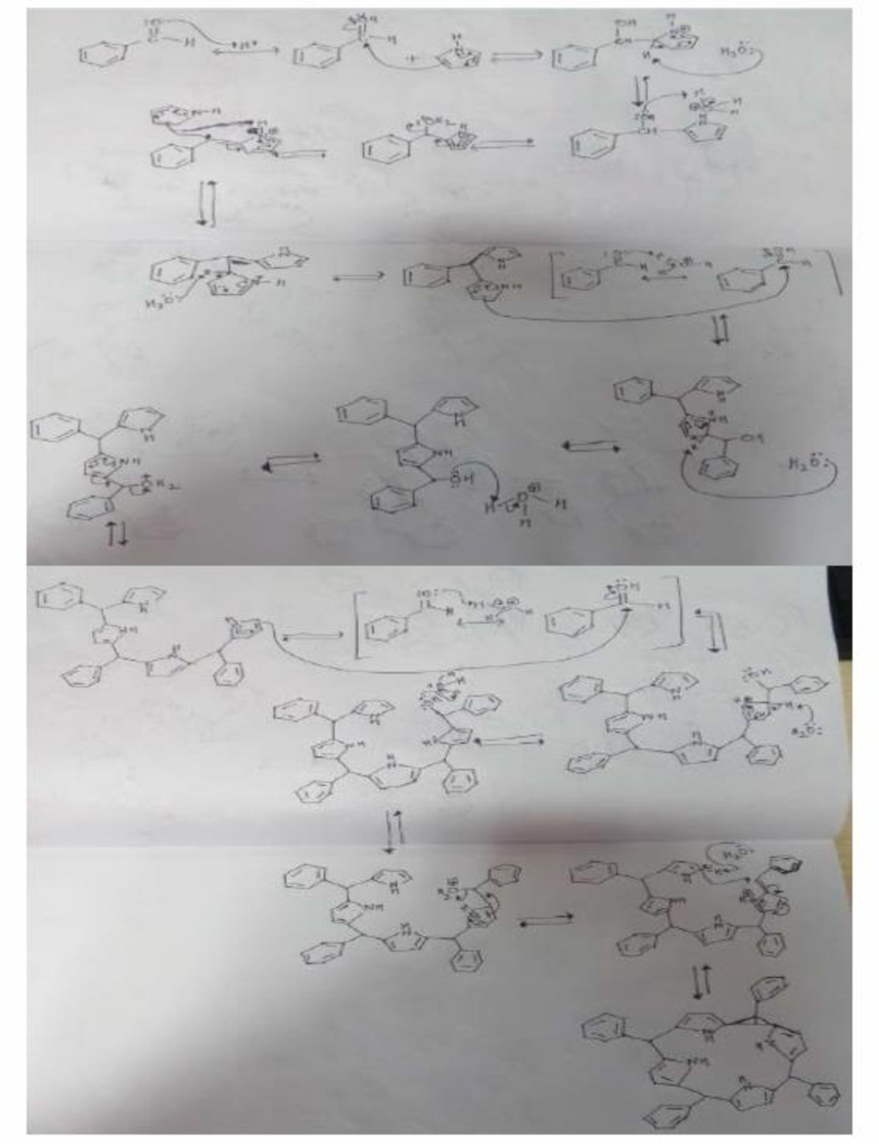
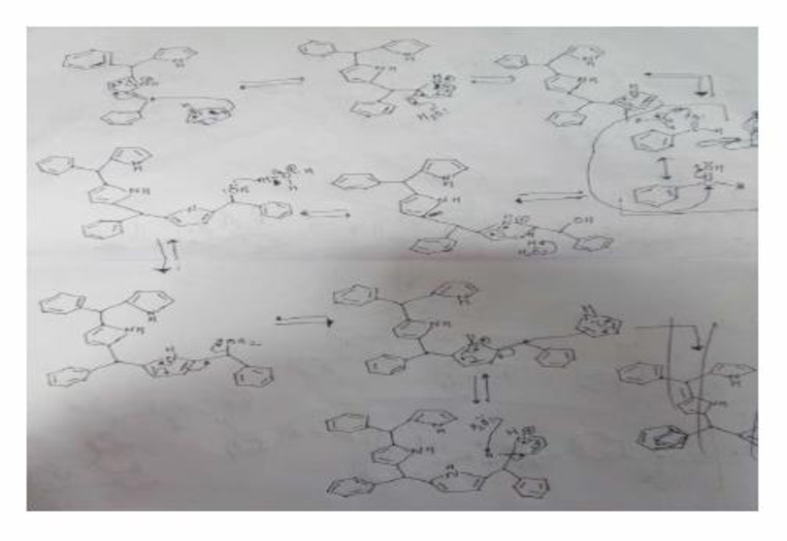
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Chemistry
Please help explain this textbook question:
Although N, N -dimethylaniline is extremely reactive toward electrophilic aromatic substitution and is readily substituted by weak electrophiles, such as diazonium and nitronium ions, this reactivity is greatly diminished by the introduction of an alkyl substituent in an ortho position.
a) Put these three common types of carbonyl compound in order of decreasing reactivity
ester amide acid chloride
b) For the least reactive, show the interconversion to its other resonance form:
How does this electron delocalisation make it stable?
c) For the most reactive, draw the mechanism of its undergoing hydrolysis (reaction with H2O):
Why makes this type of carbonyl so reactive to nucleophiles?
The reaction that occurs when the benzaldehyde you have is reacted in a basic environment is called the Cannizzaro reaction, and when it is reacted with cyanide, it is called benzoin. It Explain the reason by writing the reaction mechanisms of the reactions.
Chapter 19 Solutions
Organic Chemistry&mod Mstg Etx Vp Ac Pkg
Ch. 19.1 - Name the following:Ch. 19.2 - Prob. 3PCh. 19.2 - Prob. 4PCh. 19.3 - Draw the product of each of the following...Ch. 19.5 - Prob. 6PCh. 19.5 - Explain why cyclopentadiene (pKa = 15) is more...Ch. 19.5 - When pyrrole is added to a dilute solution of...Ch. 19.6 - Prob. 10PCh. 19.6 - How to the mechanisms of the following reactions...Ch. 19.6 - Prob. 12P
Ch. 19.6 - Rank the following compounds from easiest to...Ch. 19.7 - Prob. 14PCh. 19.7 - Prob. 15PCh. 19.7 - Prob. 16PCh. 19.7 - Prob. 17PCh. 19.7 - Prob. 18PCh. 19.7 - Prob. 19PCh. 19.7 - Prob. 20PCh. 19 - Name the following:Ch. 19 - Prob. 22PCh. 19 - Rank the following compounds from strongest acid...Ch. 19 - Which of the following compounds is easier to...Ch. 19 - Rank the following compounds from most reactive to...Ch. 19 - One of the following compounds undergoes...Ch. 19 - Benzene undergoes electrophilic aromatic...Ch. 19 - Pyrrole reacts with excess...Ch. 19 - The dipole moments of furan and tetrahydrofuran...Ch. 19 - Name the following:Ch. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - a. Draw resonance contributors to show why...Ch. 19 - The chemical shifts of the C-2 hydrogen in the...Ch. 19 - Explain why protonating aniline has a dramatic...Ch. 19 - Prob. 36PCh. 19 - Propose a mechanism for the following reaction:Ch. 19 - Prob. 38PCh. 19 - Propose a mechanism for the following reactions:Ch. 19 - Prob. 40PCh. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - Organic chemists work with tetraphenylporphyrins...Ch. 19 - Show how the following compounds can be prepared...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The widely used anticoagulant warfarin (see Chemical Connections: From Moldy Clover to a Blood Thinner in Chapter 18) is synthesized from 4-hydroxycoumarin, benzaldehyde, and acetone as shown in this retrosynthesis. Show how warfarin is synthesized from these reagents.arrow_forwardShow how to bring about each step in this synthesis of the herbicide propranil.arrow_forwardWittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forward
- 1. Put these three common types of carbonyl compound in order of decreasing reactivity ester amide acid chloride 2. For the least reactive, show the interconversion to its other resonance form: How does this electron delocalisation make it stable? 3. For the most reactive, draw the mechanism of its undergoing hydrolysis (reaction with H2O): Why makes this type of carbonyl so reactive to nucleophiles?arrow_forward1) How will you describe whether any compound has been oxidized or reduced? Support the answer with suitable examples. 2)Why carboxylic acid with a carbonyl group at 3rd position can be decarboxylated? 3) Explain why electrophilic aromatic substitution in Pyrrole takes place at C-2 positions whereas, in Pyridine it takes place at C-3 position? 4) List the following esters in order of decreasing reactivities towards hydrolysis with reason: Methyl benzoate, p-cyano methyl benzoate and p-hydroxy methyl benzoate 5)LDA is the base of choice for carbonyl compound to completely convert into enolate. Why?arrow_forwardIn biochemical reactions, decarboxylation of carboxylic acids typically takes place for-keto carboxylic acids. Justify a rational why nature opted for-keto carboxylic acid decarboxylation. Among the following types of biochemical reactions, ester hydrolysis, rearrangement reactions, water elimination reactions, and anhydride hydrolyses, which one is the most favorable one. Rank the above reactions types in the order of being the most to least favorable reactionarrow_forward
- Acid anhydrides are generally formed by strongly heating an acid solution which promotes dehydration. Intramolecular acid dehydration create cyclic acid anhydrides .i. Besides providing energy for the reaction, how else does heating the acid solution promote the formation of the anhydride? )ii. Write a mechanism for the dehydration of the following molecule (Hint:remember that cyclohexyl rings can flip)arrow_forwardWhich of the following is an example of the synthesis of the malonic ester?arrow_forwardThe Dess–Martin reaction reacts an alcohol with the Dess–Martin periodinane. The first step displaces one of the acetate groups on the periodinane with the alcohol. A base then deprotonates one of the alpha protons, forming a new carbonyl bond, iodinane and acetic acid. The oxidation stops at this stage to give the aldehyde. Both Swern and Dess–Martin conditions will also convert a secondary alcohol to a ketone. Now apply what has just been discussed. Draw the major organic product.arrow_forward
- he reaction that occurs when the benzaldehyde in your hand is reacted in a basic environment. Cannizzaro reaction is called benzoin recovery when reacted with cyanide. It Explain the reason by writing the reaction mechanisms of the reactions.arrow_forwardSuggest a synthesis of the target molecule below, using only benzene and propionyl chloride as your only carbon based reactants. you can use the benzene reactant twice in the synthesis and any inorganic reagents you wisharrow_forwardGive the structure of the starting reactant for the reaction sequence shown belowarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License