
An ideal gas is trapped inside a tube of uniform cross-sectional area sealed at one end as shown in Figure P19.49. A column of mercury separates the gas from the outside. The tube can be turned in a vertical plane. In Figure P19.49A, the column of air in the tube has length L1, whereas in Figure P19.49B, the column of air has length L2. Find an expression (in terms of the parameters given) for the length L3 of the column of air in Figure P19.49C, when the tube is inclined at an angle θ with respect to the vertical.
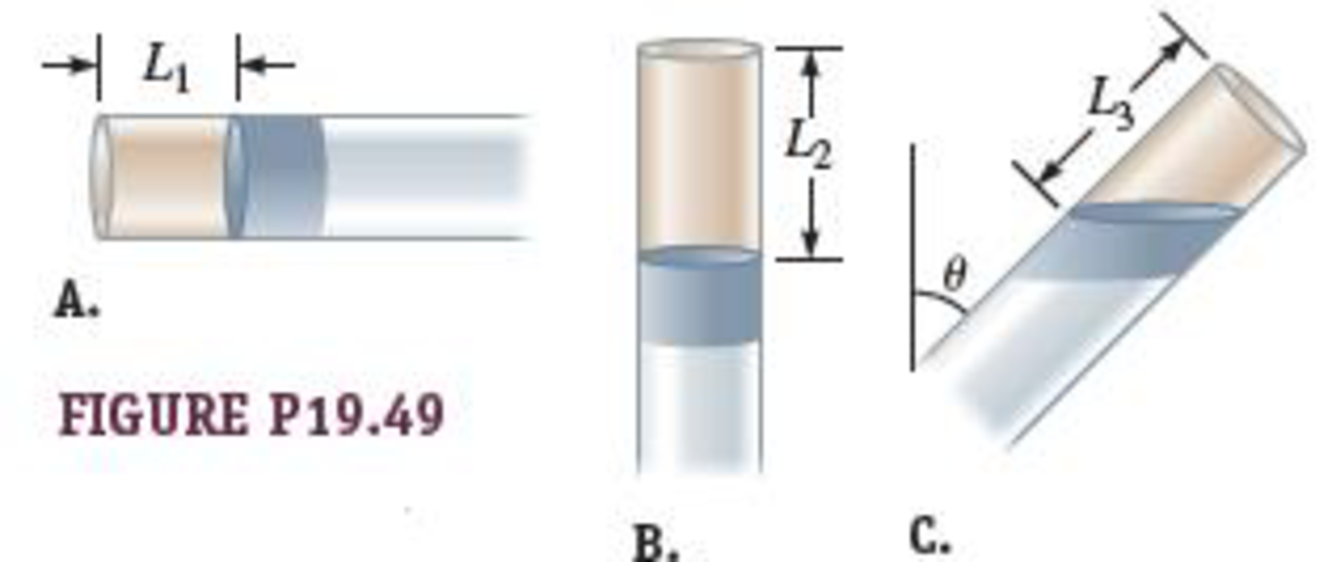
FIGURE P19.49
The expression for the length
Answer to Problem 49PQ
The expression for the length
Explanation of Solution
Three cases are depicted here. The first case in which the length of the air column is
In all the three cases mercury separates the air from outside. In all the three cases mercury should be in static equilibrium. The forces experienced by the mercury are the force due to the pressure inside the tube, the force from the atmospheric pressure, and the force due to the weight of the mercury. Here the tube is maintained at constant pressure. So apply Boyle’s law.
Consider Figure 1.
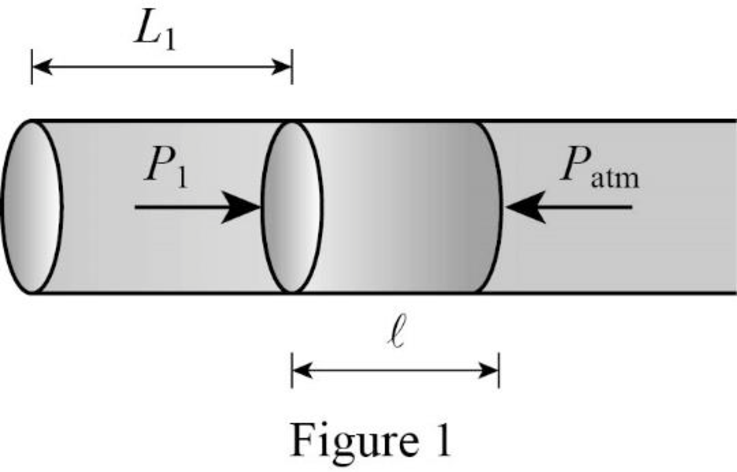
Weight of mercury acts perpendicular to the orientation of the tube. Thus the mercury is in equilibrium whenever the atmospheric pressure is equal to the pressure inside the tube.
Here,
Consider Figure 2.
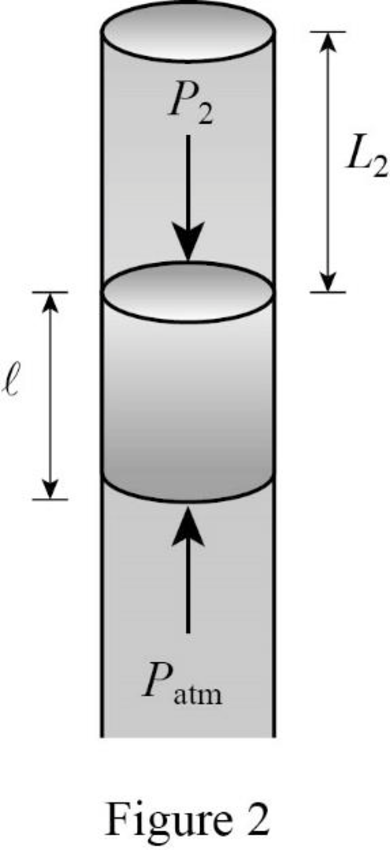
Here the outside atmospheric pressure is balanced by the sum of pressure in the tube due to the air column and the pressure due the weight of mercury.
Write the expression for the pressure due to the weight of mercury in position B.
Here,
Write the expression for the density of mercury.
Here,
Solve equation (III) for
Use expression (IV) in (II).
Here,
The atmospheric pressure at position B is balanced by the sum of pressures due to the weight of mercury, and pressure due to the column of air in the tube.
Here,
Consider the position 3.
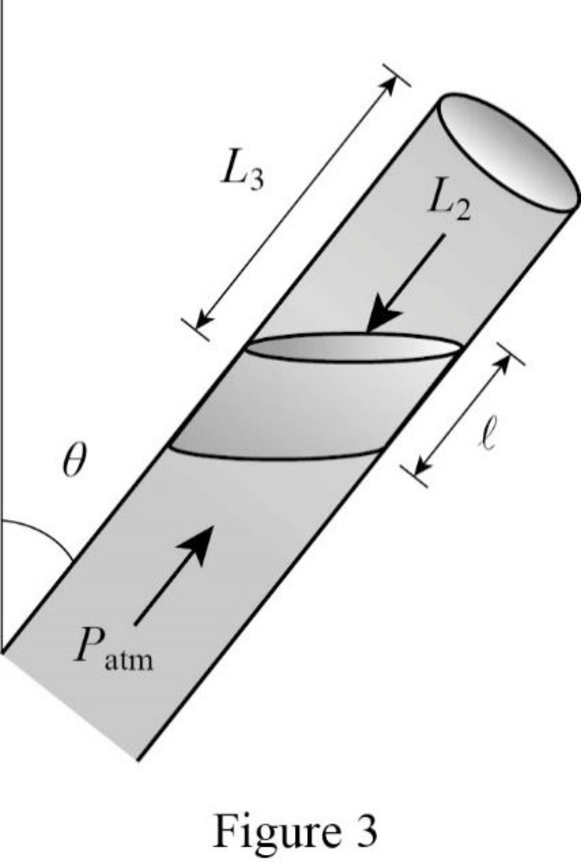
Here the pressure due to atmosphere is balanced by pressure inside the tube due to gas column denoted by
Write the expression for the vertical component of pressure due to the weight of mercury column.
Here,
Write the expression for the balance of pressure in the tube kept in position 3.
Since the temperature is constant, apply Boyle’s law. Boyle’s law states that the volume of a gas is directly proportional to the pressure of the gas at constant temperature.
Write the expression for Boyle’s law for case A and case B.
Write the expression for volume of air in tube 1.
Here,
Write the expression for volume of air in tube 2.
Here,
Use expression (XI), (VII), (XII) and (I) in expression (X).
Solve expression for
Write the expression for Boyle’s law for case A and case C.
Write the expression for volume of air in tube 3.
Here,
Use expression (XI), (XVI), (IX) and (I) in expression (XV).
Solve expression (XVII) for
Equate the right hand sides of equations (XIV) and (XVIII) and solve for
Solve expression (XIX) for
Conclusion:
Therefore, the expression for the length
Want to see more full solutions like this?
Chapter 19 Solutions
Bundle: Physics For Scientists And Engineers: Foundations And Connections, Volume 2, Loose-leaf Version + Webassign Printed Access Card For Katz's ... And Connections, Single-term Courses
- A gas is in a container of volume V0 at pressure P0. It is being pumped out of the container by a piston pump. Each stroke of the piston removes a volume Vs through valve A and then pushes the air out through valve B as shown in Figure P19.74. Derive an expression that relates the pressure Pn of the remaining gas to the number of strokes n that have been applied to the container. FIGURE P19.74arrow_forwardA vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P16.56). The piston is not restricted in its motion in any way and is supported by the gas at pressure P below it. Atmospheric pressure is P0. We wish to find die height h in Figure P16.56. (a) What analysis model is appropriate to describe the piston? (b) Write an appropriate force equation for the piston from this analysis model in terms of P, P0, m, A, and g. (c) Suppose n moles of an ideal gas are in the cylinder at a temperature of T. Substitute for P in your answer to part (b) to find the height h of the piston above the bottom of the cylinder.arrow_forwardA cylinder that has a 40.0-cm radius and is 50.0 cm deep is filled with air at 20.0C and 1.00 atm (Fig. P10.74a). A 20.0-kg piston is now lowered into the cylinder, compressing the air trapped inside as it takes equilibrium height hi (Fig. P16.74b). Finally, a 25.0-kg dog stands on the piston, further compressing the air, which remains at 20C (Fig. P16.74c). (a) How far down (h) does the piston move when the dog steps onto it? (b) To what temperature should the gas be warmed to raise the piston and dog back to hi?arrow_forward
- Review. (a) Derive an expression for the buoyant force on a spherical balloon, submerged in water, as a function of the depth h below the surface, the volume Vi of the balloon at the surface, the pressure P0 at the surface, and the density w of the water. Assume the water temperature does not change with depth, (b) Does the bouyant force increase or decrease as the balloon is submerged? (c) At what depth is the buoyant force one-half the surface value?arrow_forward(a) An ideal gas occupies a volume of 1.0 cm3 at 20.C and atmospheric pressure. Determine the number of molecules of gas in the container, (b) If the pressure of the 1.0-cm3 volume is reduced to 1.0 1011 Pa (an extremely good vacuum) while the temperature remains constant, how many moles of gas remain in the container?arrow_forwardA sealed cubical container 20.0 cm on a side contains a gas with three times Avogadros number of neon atoms at a temperature of 20.0C. (a) Find the internal energy of the gas. (b) Find the total translational kinetic energy of the gas. (c) Calculate the average kinetic energy per atom, (d) Use Equation 10.13 to calculate the gas pressure. (e) Calculate the gas pressure using the ideal gas law (Eq. 10.8).arrow_forward
- (a) Given that air is 21% oxygen, find the minimum atmospheric pressure that gives a relatively safe partial pressure of oxygen of 0.16 atm. (b) What is the minimum pressure that gives a partial pressure of oxygen above the quickly fatal level of 0.06 atm? (c) The air pressure at the summit of Mount Everest (8848 m) is 0.334 atm. Why have a few people climbed it without oxygen, while some who have tried, even though they had trained at high elevation, had to tum back?arrow_forwardA cylinder with a piston holds 0.50 m3 of oxygen at an absolute pressure of 4.0 atm. The piston is pulled outward, increasing the volume of the gas until the pressure drops to 1.0 atm. If the temperature stays constant, what new volume does the gas occupy? (a) 1.0 m3 (b) 1.5 m3 (c) 2.0 m3 (d) 0.12 m3 (e) 2.5 m3arrow_forwardA 40.0-g projectile is launched by the expansion of hot gas in an arrangement shown in Figure P12.4a. The cross sectional area of the launch tube is 1.0 cm2, and the length that the projectile travels down the tube after starting from rest is 52 cm. As the gas expands, the pressure varies as shown in Figure P12.4b. The values for the initial pressure and volume are P1 = 11 105 Pa and Vi = 8.0 cm3 while the final values are Pf = 1.0 105 Pa and Vf = 8.0 cm3. Friction between the projectile and the launch tube is negligible, (a) If the projectile is launched into a vacuum, what is the speed of the projectile as it leaves the launch tube? (b) If instead the projectile is launched into air at a pressure of 1.0 105 Pa. what fraction of the work done by the expanding gas in the tube is spent by the projectile pushing air out of the way as it proceeds down tile tube?arrow_forward
- A manometer containing water with one end connected to a container of gas has a column height difference of 0.60 m (Fig. P15.72). If the atmospheric pressure on the right column is 1.01 105 Pa, find the absolute pressure of the gas in the container. The density of water is 1.0 103 kg/m3. FIGURE P15.72arrow_forward(a) Show that the density of an ideal gas occupying a volume V is given by = PM/KT, where M is the molar mass. (b) Determine the density of oxygen gas at atmospheric pressure and 20.0C.arrow_forwardConsider the piston cylinder apparatus shown in Figure P20.81. The bottom of the cylinder contains 2.00 kg of water at just under 100.0c. The cylinder has a radius of r = 7.50 cm. The piston of mass m = 3.00 kg sits on the surface of the water. An electric heater in the cylinder base transfers energy into the water at a rate of 100 W. Assume the cylinder is much taller than shown in the figure, so we dont need to be concerned about the piston reaching the top of the cylinder. (a) Once the water begins boiling, how fast is the piston rising? Model the steam as an ideal gas. (b) After the water has completely turned to steam and the heater continues to transfer energy to the steam at the same rate, how fast is the piston rising?arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





