
Concept explainers
A cylinder that has a 40.0-cm radius and is 50.0 cm deep is filled with air at 20.0°C and 1.00 atm (Fig. P10.74a). A 20.0-kg piston is now lowered into the cylinder, compressing the air trapped inside as it takes equilibrium height hi (Fig. P16.74b). Finally, a 25.0-kg dog stands on the piston, further compressing the air, which remains at 20°C (Fig. P16.74c). (a) How far down (Δh) does the piston move when the dog steps onto it? (b) To what temperature should the gas be warmed to raise the piston and dog back to hi?
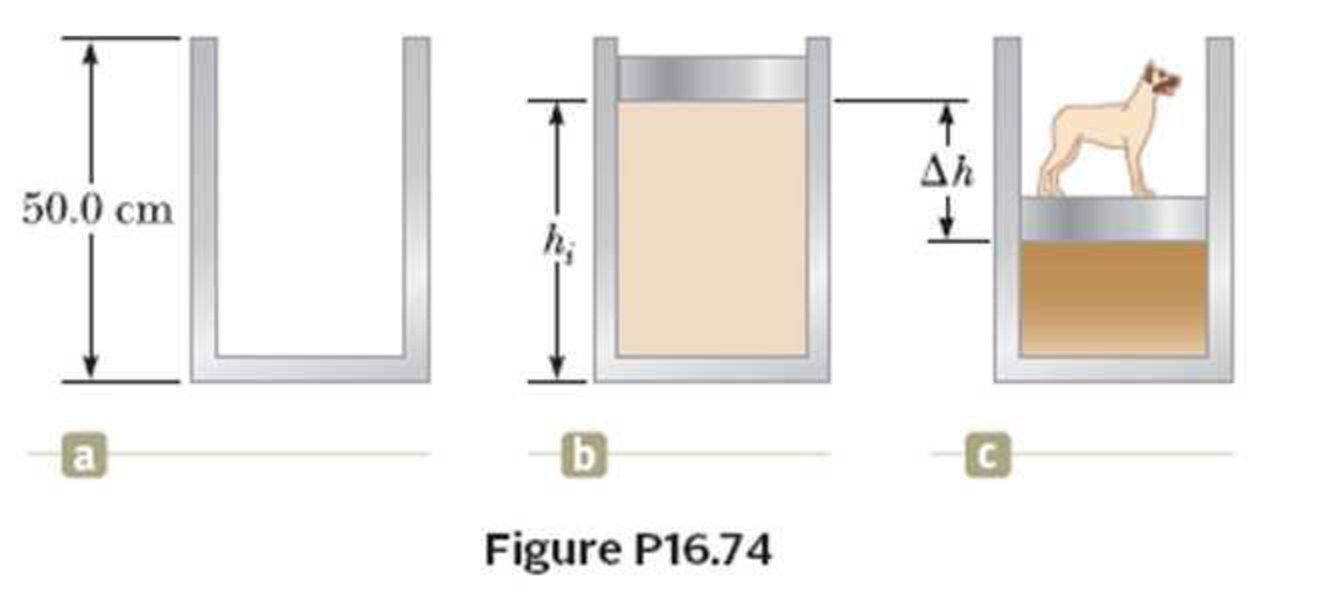
(a)
The height which the piston moves down when the dog steps onto the piston.
Answer to Problem 74P
The height at which the piston move down when the dog steps onto the piston is
Explanation of Solution
Write the expression for Boyle’s law, for the initial and final state since the temperature is constant.
Here,
Use
Here,
When the piston moves down, then the external pressure on the piston is
Equate the equation (II) with the equation
Replace the mass
Here,
The piston moves downward when the dog steps on the piston. It can be expressed as,
Use equation (III) and (IV) in (V).
Conclusion:
Substitute,
Therefore, the height at which the piston move down when the dog steps onto the piston is
(b)
The temperature at which the gas should be warmed to raise the piston and dog back to
Answer to Problem 74P
The temperature at which the gas should be warmed to raise the piston and dog back to
Explanation of Solution
Write the expression for Charles’s law, for the initial and final state since the pressure is constant.
Here,
Use
Use equation (III) and (IV) in the equation (VIII).
Conclusion:
Substitute,
Therefore, The temperature at which the gas should be warmed to raise the piston and dog back to
Want to see more full solutions like this?
Chapter 16 Solutions
Principles of Physics: A Calculus-Based Text
- A 40.0-g projectile is launched by the expansion of hot gas in an arrangement shown in Figure P12.4a. The cross sectional area of the launch tube is 1.0 cm2, and the length that the projectile travels down the tube after starting from rest is 52 cm. As the gas expands, the pressure varies as shown in Figure P12.4b. The values for the initial pressure and volume are P1 = 11 105 Pa and Vi = 8.0 cm3 while the final values are Pf = 1.0 105 Pa and Vf = 8.0 cm3. Friction between the projectile and the launch tube is negligible, (a) If the projectile is launched into a vacuum, what is the speed of the projectile as it leaves the launch tube? (b) If instead the projectile is launched into air at a pressure of 1.0 105 Pa. what fraction of the work done by the expanding gas in the tube is spent by the projectile pushing air out of the way as it proceeds down tile tube?arrow_forwardA vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P16.56). The piston is not restricted in its motion in any way and is supported by the gas at pressure P below it. Atmospheric pressure is P0. We wish to find die height h in Figure P16.56. (a) What analysis model is appropriate to describe the piston? (b) Write an appropriate force equation for the piston from this analysis model in terms of P, P0, m, A, and g. (c) Suppose n moles of an ideal gas are in the cylinder at a temperature of T. Substitute for P in your answer to part (b) to find the height h of the piston above the bottom of the cylinder.arrow_forwardA vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P18.40). The piston is not restricted in its motion in any way and is supported by the gas at pressure P below it. Atmospheric pressure is P0. We wish to find the height h in Figure P18.40. (a) What analysis model is appropriate to describe the piston? (b) Write an appropriate force equation for the piston from this analysis model in terms of P, P0, m, A, and g. (c) Suppose n moles of an ideal gas are in the cylinder at a temperature of T. Substitute for P in your answer to part (b) to find the height h of the piston above the bottom of the cylinder. Figure P18.40arrow_forward
- An ideal gas is trapped inside a tube of uniform cross-sectional area sealed at one end as shown in Figure P19.49. A column of mercury separates the gas from the outside. The tube can be turned in a vertical plane. In Figure P19.49A, the column of air in the tube has length L1, whereas in Figure P19.49B, the column of air has length L2. Find an expression (in terms of the parameters given) for the length L3 of the column of air in Figure P19.49C, when the tube is inclined at an angle with respect to the vertical. FIGURE P19.49arrow_forwardA gas is in a container of volume V0 at pressure P0. It is being pumped out of the container by a piston pump. Each stroke of the piston removes a volume Vs through valve A and then pushes the air out through valve B as shown in Figure P19.74. Derive an expression that relates the pressure Pn of the remaining gas to the number of strokes n that have been applied to the container. FIGURE P19.74arrow_forward(a) An ideal gas occupies a volume of 1.0 cm3 at 20.C and atmospheric pressure. Determine the number of molecules of gas in the container, (b) If the pressure of the 1.0-cm3 volume is reduced to 1.0 1011 Pa (an extremely good vacuum) while the temperature remains constant, how many moles of gas remain in the container?arrow_forward
- (a) Show that the density of an ideal gas occupying a volume V is given by = PM/KT, where M is the molar mass. (b) Determine the density of oxygen gas at atmospheric pressure and 20.0C.arrow_forwardOne cylinder contains helium gas and another contains krypton gas at the same temperature. Mark each of these statements true, false, or impossible to determine from the given information. (a) The rms speeds of atoms in the two gases are the same. (b) The average kinetic energies of atoms in the two gases are the same. (c) The internal energies of 1 mole of gas in each cylinder are the same. (d) The pressures in the two cylinders ale the same.arrow_forwardA metallic container of fixed volume of 2.5103 m3 immersed in a large tank of temperature 27 contains two compartments separated by a freely movable wall. Initially, the wall is kept in place by a stopper so that there are 0.02 mol of the nitrogen gas on one side and 0.03 mol of the oxygen gas on the other side, each occupying half the volume. When the stopper is removed, the wall moves and comes to a final position. The movement of the wall is controlled so that the wall moves in infinitesimal quasi-static steps. (a) Find the final volumes of the two sides assuming the ideal gas behavior for the two gases. (b) How much work does each gas do on the other? (c) What is the change in the internal energy of each gas? (d) Find the amount of heat that enters or leaves each gas.arrow_forward
- Cylinder A contains oxygen (O2) gas, and cylinder B contains nitrogen (N2) gas. If the molecules in the two cylinders have the same rms speeds, which of the following statements is false? (a) The two gases haw different temperatures. (b) The temperature of cylinder B is less than the temperature of cylinder A. (c) The temperature of cylinder B is greater than the temperature of cylinder A. (d) The average kinetic energy of the nitrogen molecules is less than the average kinetic energy of the oxygen molecules.arrow_forward(a) An ideal gas occupies a volume of 1.0 cm3 at 20.C and atmospheric pressure. Determine the number of molecules of gas in the container, (b) If the pressure of the 1.0-cm3 volume is reduced to 1.0 1011 Pa (an extremely good vacuum) while the temperature remains constant, how many moles of gas remain in the container?arrow_forwardA cylinder with a piston holds 0.50 m3 of oxygen at an absolute pressure of 4.0 atm. The piston is pulled outward, increasing the volume of the gas until the pressure drops to 1.0 atm. If the temperature stays constant, what new volume does the gas occupy? (a) 1.0 m3 (b) 1.5 m3 (c) 2.0 m3 (d) 0.12 m3 (e) 2.5 m3arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning





