
(a)
The PV diagram for the cycle.
(a)
Answer to Problem 84P
The required PV diagram is shown in Figure 1.
Explanation of Solution
Formula:
The range of the isobaric expansion cycle is given by,
The range of the adiabatic compression cycle is given by,
The range for the isobaric compression cycle is given by,
The range of the adiabatic expansion cycle is given by,
Calculation:
The state functions A for the PV graph are given by,
The state functions B for the PV graph are given by,
The state functions C for the PV graph are given by,
The state functions D for the PV graph are given by,
The required PV diagram is shown in Figure 1
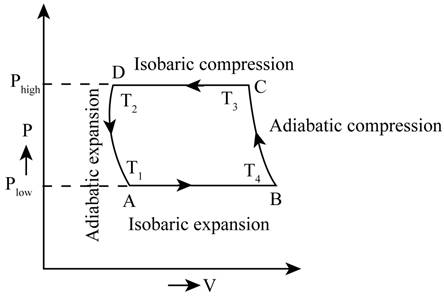
Figure 1
Conclusion:
Therefore, the required PV diagram is shown in Figure 1
(b)
The expression for the coefficient of performance.
(b)
Answer to Problem 84P
The value of
Explanation of Solution
Formula Used:
The expression for the coefficient of performance is given by,
The expression for
The expression for the value of
The expression for the work done during the cycle is given by,
The expression for the heat released in the entire cycle is given by,
The expression to determine the value of
The expression to determine the value of
Calculation:
The expression for the value of the
The difference between the value of
The value of
Conclusion:
Therefore, the value of
(c)
The coefficient of performance of the refrigerator.
(c)
Answer to Problem 84P
The value of value of
Explanation of Solution
Given:
The volume
The initial pressure
The volume
The temperature
The pressure ratio
Formula:
The expression for the temperature and the pressure after adiabatic process BC is given by,
The expression for the temperature and the pressure after adiabatic process DA is given by,
From above and from equation (1) the value of
The expression for
Calculation:
The value of
Conclusion:
Therefore, the value of
(d)
The electrical energy that must be supplied to the motor of rotor is given by,
(d)
Answer to Problem 84P
The electrical energy that must be supplied is
Explanation of Solution
Given:
The rate
Formula:
The expression to determine the electrical energy that must be supplied is given by,
Calculation:
The electrical energy that must be supplied is calculated as,
Conclusion:
Therefore, the electrical energy that must be supplied is
(e)
The amount added to the monthly electricity bill.
(e)
Answer to Problem 84P
The electrical energy that must be supplied is
Explanation of Solution
Given:
The number of days
The time for which the motor runs is
The charge
Formula:
The amount of electrical energy utilized by the refrigerator per month is given by,
Calculation:
The electrical energy utilized by the refrigerator per month is calculated as,
Conclusion:
Therefore, the electrical energy that must be supplied is
Want to see more full solutions like this?
Chapter 19 Solutions
Physics for Scientists and Engineers
- In a diesel engine, the fuel is ignited without a spark plug. Instead, air in a cylinder is compressed adiabatically to a temperature above the ignition temperature of the fuel; at the point of maximum compression, the fuel is injected into the cylinder. Suppose that air at 20 C is taken into the cylinder at a volume V1 and then compressed adiabatically and quasi-statically to a temperature of 600 C and a volume V2 . If =1.4 , what is the ratio V1/V2 ? (Note: static. In an operating diesel engine, the compression is not quasi-arrow_forwardWhich of the following is true for the entropy change of a system that undergoes a reversible, adiabatic process? (a) S 0 (b) S = 0 (c) S 0arrow_forwardTwo moles of a monatomic ideal gas such as oxygen is compressed adiabatically and reversibly from a state (3 atm, 5 L) to a state with a pressure of 4 atm. (a) Find the volume and temperature of the final state. (b) Find the temperature of the initial state. (c) Find work done by the gas in the process. (d) Find the change in internal energy in the process. Assume Cv=5R and Cp=Cv+R for the diatomic ideal gas in the conditions given.arrow_forward
- What is the entropy change of 10 g of steam at 100 when it condenses to water at the same temperature?arrow_forwardIs it possible for a system to have an entropy change if it neither absorbs nor emits heat during a reversible? transition? What happens it the process is irreversible?arrow_forwardThe energy input to an engine is 3.00 times greater than the work it performs. (i) What is its thermal efficiency? (a) 3.00 (b) 1.00 (c) 0.333 (d) impossible to determine (ii) What fraction of the energy input is expelled to the cold reservoir? (a) 0.333 (b) 0.667 (c) 1.00 (d) impossible to determinearrow_forward
- A monatomic ideal gas undergoes a quasi-static adiabatic expansion in which its volume is doubled. How is the pressure of the gas changed?arrow_forwardA copper rod of cross-sectional area 5.0 cm2 and length 5.0 m conducts heat from a heat reservoir at 373 K to one at 273 K. What is the time rate of change of the universe's entropy for this process?arrow_forwardAn ideal heat pump, one that satisfies the Carnot relationship W = Qh11 - Tc>Th2, is used to heata room that is at 293 K. If the pump does 275 J of work, how much heat does it supply to the room ifthe outdoor temperature is (a) 273 K or (b) 263 K?arrow_forward
- The ideal gas in a Carnot engine extracts 800 J of heat energy during the isothermal expansion at 260 ∘C. How much heat energy is exhausted during the isothermal compression at 50 ∘C?arrow_forwardIn a very mild winter climate, a heat pump has heat transfer from an environment at 5.00C to one at 35.0C. What is the best possible coefficient of performance for these temperatures? Explicitly show how you follow the steps in the Problem-Solving Strategies for Thermodynamics.arrow_forwardImagine a Carnot heat pump operates between an outside temperature of 0 °C and an inside temperature of 20.0 °C . What is the work needed if the heat delivered to the inside of the house is 30.0 kJ?StrategyBecause the heat pump is assumed to be a Carnot pump, its performance coefficient is given by KP = Qh /W = Th /(Th − Tc). Thus, we can find the work W from the heat delivered Qh.arrow_forward

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


